Effects of Porous Structure Development and Ash on the Steam Gasification Reactivity of Biochar Residues from a Commercial Gasifier at Different Temperatures
Abstract
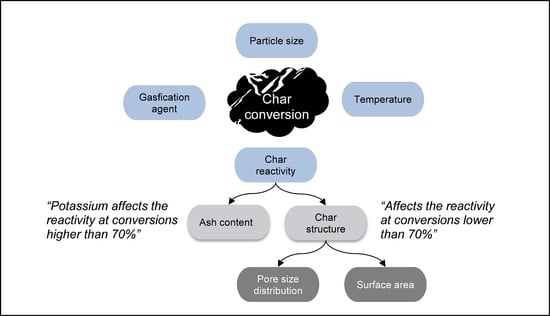
:1. Introduction
2. Method
2.1. Raw Material and Sample Preparation
2.2. Steam Char Gasification TGA Experiments
2.3. Textural Analysis (N2 Physisorption)
2.4. SEM Analysis
3. Results
3.1. Original Char
3.2. Water Leached Chars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acharya, B.; Dutta, A.; Basu, P. An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO. Int. J. Hydrogen Energy 2010, 35, 1582–1589. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. WIREs Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Gupta, A.K. Kinetics of woodchips char gasification with steam and carbon dioxide. Appl. Energy 2011, 88, 1613–1619. [Google Scholar] [CrossRef]
- Laurendeau, N.M. Heterogeneous kinetics of coal char gasification and combustion. Prog. Energy Combust. Sci. 1978, 4, 221–270. [Google Scholar] [CrossRef]
- Smith, I.W. The combustion rates of coal chars: A review. Symp. (Int.) Combust. 1982, 19, 1045–1065. [Google Scholar] [CrossRef]
- Bews, I.M.; Hayhurst, A.N.; Richardson, S.M.; Taylor, S.G. The order, Arrhenius parameters, and mechanism of the reaction between gaseous oxygen and solid carbon. Combust. Flame 2001, 124, 231–245. [Google Scholar] [CrossRef]
- Hurt, R.H.; Calo, J.M. Semi-global intrinsic kinetics for char combustion modeling. Combust. Flame 2001, 125, 1138–1149. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Rodríguez, J.J.; Ramiro, M.J. Catalysed and uncatalysed steam gasification of eucalyptus char: Influence of variables and kinetic study. Fuel 2001, 80, 2025–2036. [Google Scholar] [CrossRef]
- Di Blasi, C. Combustion and gasification rates of lignocellulosic chars. Prog. Energy Combust. Sci. 2009, 35, 121–140. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef] [Green Version]
- Septien, S.; Escudero Sanz, F.J.; Salvador, S.; Valin, S. The effect of pyrolysis heating rate on the steam gasification reactivity of char from woodchips. Energy 2018, 142, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Avila, C.; Pang, C.H.; Wu, T.; Lester, E. Morphology and reactivity characteristics of char biomass particles. Bioresour. Technol. 2011, 102, 5237–5243. [Google Scholar] [CrossRef]
- Mermoud, F.; Salvador, S.; Van de Steene, L.; Golfier, F. Influence of the pyrolysis heating rate on the steam gasification rate of large wood char particles. Fuel 2006, 85, 1473–1482. [Google Scholar] [CrossRef] [Green Version]
- Senneca, O. Kinetics of pyrolysis, combustion and gasification of three biomass fuels. Fuel Process. Technol. 2007, 88, 87–97. [Google Scholar] [CrossRef]
- Gil, M.V.; Riaza, J.; Álvarez, L.; Pevida, C.; Rubiera, F. Biomass devolatilization at high temperature under N2 and CO2: Char morphology and reactivity. Energy 2015, 91, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Wang, Y.; Zhu, S.; Li, F.; Xie, K. Structural features and gasification reactivity of coal chars formed in Ar and CO2 atmospheres at elevated pressures. Energy 2014, 74, 464–470. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Gadiou, R.; Escudero Sanz, F.J.; Salvador, S. Biomass char gasification by H2O, CO2 and their mixture: Evolution of chemical, textural and structural properties of the chars. Energy 2016, 112, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Hu, S.; Xiang, J.; Yi, W.; Bai, X.; Sun, L.; Su, S. Evolution of char structure during steam gasification of the chars produced from rapid pyrolysis of rice husk. Bioresour. Technol. 2012, 114, 691–697. [Google Scholar] [CrossRef]
- Keown, D.M.; Hayashi, J.-I.; Li, C.-Z. Drastic changes in biomass char structure and reactivity upon contact with steam. Fuel 2008, 87, 1127–1132. [Google Scholar] [CrossRef]
- Wu, H.; Yip, K.; Tian, F.; Xie, Z.; Li, C.-Z. Evolution of Char Structure during the Steam Gasification of Biochars Produced from the Pyrolysis of Various Mallee Biomass Components. Ind. Eng. Chem. Res. 2009, 48, 10431–10438. [Google Scholar] [CrossRef]
- Kajita, M.; Kimura, T.; Norinaga, K.; Li, C.-Z.; Hayashi, J.-I. Catalytic and Noncatalytic Mechanisms in Steam Gasification of Char from the Pyrolysis of Biomass. Energy Fuels 2010, 24, 108–116. [Google Scholar] [CrossRef]
- Bai, Y.; Lv, P.; Yang, X.; Gao, M.; Zhu, S.; Yan, L.; Li, F. Gasification of coal char in H2O/CO2 atmospheres: Evolution of surface morphology and pore structure. Fuel 2018, 218, 236–246. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Evolution of biomass char features and their role in the reactivity during steam gasification in a conical spouted bed reactor. Energy Convers. Manag. 2019, 181, 214–222. [Google Scholar] [CrossRef]
- Davidsson, K.O.; Korsgren, J.G.; Pettersson, J.B.C.; Jäglid, U. The effects of fuel washing techniques on alkali release from biomass. Fuel 2002, 81, 137–142. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; D’Errico, G. Degradation characteristics of straw and washed straw. Thermochim. Acta 2000, 364, 133–142. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Bakker, R.R.; Wei, J.B. On the properties of washed straw. Biomass Bioenergy 1996, 10, 177–200. [Google Scholar] [CrossRef]
- Zolin, A.; Jensen, A.; Jensen, P.A.; Frandsen, F.; Dam-Johansen, K. The Influence of Inorganic Materials on the Thermal Deactivation of Fuel Chars. Energy Fuels 2001, 15, 1110–1122. [Google Scholar] [CrossRef]
- Aho, A.; DeMartini, N.; Pranovich, A.; Krogell, J.; Kumar, N.; Eränen, K.; Holmbom, B.; Salmi, T.; Hupa, M.; Murzin, D.Y. Pyrolysis of pine and gasification of pine chars-influence of organically bound metals. Bioresour. Technol. 2013, 128, 22–29. [Google Scholar] [CrossRef]
- Bouraoui, Z.; Dupont, C.; Jeguirim, M.; Limousy, L.; Gadiou, R. CO2 gasification of woody biomass chars: The influence of K and Si on char reactivity. C. R. Chim. 2016, 19, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yin, X.; Wu, C.; Wang, C.; Xie, J.; Zhou, Z.; Ma, L.; Li, H. Effects of metal catalysts on CO2 gasification reactivity of biomass char. Biotechnol. Adv. 2009, 27, 568–572. [Google Scholar] [CrossRef]
- Karlström, O.; Dirbeba, M.J.; Costa, M.; Brink, A.; Hupa, M. Influence of K/C Ratio on Gasification Rate of Biomass Chars. Energy Fuels 2018, 32, 10695–10700. [Google Scholar] [CrossRef]
- Perander, M.; DeMartini, N.; Brink, A.; Kramb, J.; Karlström, O.; Hemming, J.; Moilanen, A.; Konttinen, J.; Hupa, M. Catalytic effect of Ca and K on CO2 gasification of spruce wood char. Fuel 2015, 150, 464–472. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakajima, H.; Ikenaga, N.-O.; Oda, H.; Miyake, T. Effect of mineral matters in biomass on the gasification rate of their chars. Biomass Conv. Bioref. 2011, 1, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kirtania, K.; Axelsson, J.; Matsakas, L.; Christakopoulos, P.; Umeki, K.; Furusjö, E. Kinetic study of catalytic gasification of wood char impregnated with different alkali salts. Energy 2017, 118, 1055–1065. [Google Scholar] [CrossRef]
- Kelebopile, L.; Sun, R.; Liao, J. Fly ash and coal char reactivity from Thermo-gravimetric (TGA) experiments. Fuel Process. Technol. 2011, 92, 1178–1186. [Google Scholar] [CrossRef]
- Klinghoffer, N.B.; Castaldi, M.J.; Nzihou, A. Influence of char composition and inorganics on catalytic activity of char from biomass gasification. Fuel 2015, 157, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Wang, Z.; Yu, Z.; Zhang, Q.; Li, C.; Fang, Y. Experimental and Kinetic Investigations of CO2 Gasification of Fine Chars Separated from a Pilot-Scale Fluidized-Bed Gasifier. Energy Fuels 2013, 27, 2422–2430. [Google Scholar] [CrossRef]
- Gu, J.; Wu, S.; Wu, Y.; Li, Y.; Gao, J. Differences in Gasification Behaviors and Related Properties between Entrained Gasifier Fly Ash and Coal Char. Energy Fuels 2008, 22, 4029–4033. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Z.; Dong, Q.; Yu, K.; Lu, Q. Structural Properties and Gasification Reactivity of Shenmu Fly Ash Obtained from a 5t/d Circulating Fluidized Bed Gasifier. Procedia Eng. 2015, 102, 1104–1111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, H.; Zhu, Z. Regasification properties of industrial CFB-gasified semi-char. J. Therm. Anal. Calorim. 2018, 131, 3035–3046. [Google Scholar] [CrossRef]
- Meng, X.; Benito, P.; de Jong, W.; Basile, F.; Verkooijen, A.H.M.; Fornasari, G.; Vaccari, A. Steam–O2 Blown Circulating Fluidized-Bed (CFB) Biomass Gasification: Characterization of Different Residual Chars and Comparison of Their Gasification Behavior to Thermogravimetric (TG)-Derived Pyrolysis Chars. Energy Fuels 2012, 26, 722–739. [Google Scholar] [CrossRef]
- Marquez-Montesinos, F.; Cordero, T.; Rodrıguez-Mirasol, J.; Rodrıguez, J.J. CO2 and steam gasification of a grapefruit skin char. Fuel 2002, 81, 423–429. [Google Scholar] [CrossRef]
- Dupont, C.; Jacob, S.; Marrakchy, K.O.; Hognon, C.; Grateau, M.; Labalette, F.; Da Silva Perez, D. How inorganic elements of biomass influence char steam gasification kinetics. Energy 2016, 109, 430–435. [Google Scholar] [CrossRef]
- Dupont, C.; Nocquet, T.; Da Costa, J.A.; Verne-Tournon, C. Kinetic modelling of steam gasification of various woody biomass chars: Influence of inorganic elements. Bioresour. Technol. 2011, 102, 9743–9748. [Google Scholar] [CrossRef] [PubMed]
- Hognon, C.; Dupont, C.; Grateau, M.; Delrue, F. Comparison of steam gasification reactivity of algal and lignocellulosic biomass: Influence of inorganic elements. Bioresour. Technol. 2014, 164, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.; Tian, F.; Hayashi, J.-I.; Wu, H. Effect of Alkali and Alkaline Earth Metallic Species on Biochar Reactivity and Syngas Compositions during Steam Gasification. Energy Fuels 2010, 24, 173–181. [Google Scholar] [CrossRef]
- Ohki, A.; Nakajima, T.; Yamashita, H.; Iwashita, A.; Takanashi, H. Leaching of various metals from coal into aqueous solutions containing an acid or a chelating agent. Fuel Process. Technol. 2004, 85, 1089–1102. [Google Scholar] [CrossRef]
- Haul, R. S. J. Gregg, K. S. W. Sing: Adsorption, Surface Area and Porosity. 2. Auflage, Academic Press, London 1982. 303 Seiten, Preis: 49.50. Berichte der Bunsengesellschaft für Physikalische Chemie 1982, 86, 957. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Defoort, F.; Dupont, C.; Durruty, J.; Guillaudeau, J.; Bedel, L.; Ravel, S.; Campargue, M.; Labalette, F.; Da Silva Perez, D. Thermodynamic Study of the Alkali Release Behavior during Steam Gasification of Several Biomasses. Energy Fuels 2015, 29, 7242–7253. [Google Scholar] [CrossRef]
- Moud, P.H.; Andersson, K.J.; Lanza, R.; Pettersson, J.B.C.; Engvall, K. Effect of gas phase alkali species on tar reforming catalyst performance: Initial characterization and method development. Fuel 2015, 154, 95–106. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Y.; Guo, P.; Kong, J.; Wu, Y.; Chang, L.; Wang, J.; Xie, W. A comparative study on the intrinsic reactivity and structural evolution during gasification of chars from biomass and different rank coals. J. Anal. Appl. Pyrolysis 2020, 149, 104859. [Google Scholar] [CrossRef]
- Ashu, J.T.; Nsakala, N.Y.; Mahajan, O.P.; Walker, P.L. Enhancement of char reactivity by rapid heating of precursor coal. Fuel 1978, 57, 250–251. [Google Scholar] [CrossRef]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T.F. Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Gomi, K.; Hishinuma, Y. Effect of pre-oxidation on reactivity of chars in steam. Fuel 1982, 61, 77–80. [Google Scholar] [CrossRef]
- Tremel, A.; Haselsteiner, T.; Nakonz, M.; Spliethoff, H. Coal and char properties in high temperature entrained flow gasification. Energy 2012, 45, 176–182. [Google Scholar] [CrossRef]
- Fatehi, H.; Bai, X.-S. Structural evolution of biomass char and its effect on the gasification rate. Appl. Energy 2017, 185, 998–1006. [Google Scholar] [CrossRef]
- Struis, R.P.W.J.; Von Scala, C.; Stucki, S.; Prins, R. Gasification reactivity of charcoal with CO2. Part I: Conversion and structural phenomena. Chem. Eng. Sci. 2002, 57, 3581–3592. [Google Scholar] [CrossRef]
- Hodge, E.M.; Roberts, D.G.; Harris, D.J.; Stubington, J.F. The Significance of Char Morphology to the Analysis of High-Temperature Char−CO2 Reaction Rates. Energy Fuels 2010, 24, 100–107. [Google Scholar] [CrossRef]
- Naredi, P.; Pisupati, S.V. Interpretation of Char Reactivity Profiles Obtained Using a Thermogravimetric Analyzer. Energy Fuels 2008, 22, 317–320. [Google Scholar] [CrossRef]
- Guizani, C.; Escudero Sanz, F.J.; Salvador, S. Influence of temperature and particle size on the single and mixed atmosphere gasification of biomass char with H2O and CO2. Fuel Process. Technol. 2015, 134, 175–188. [Google Scholar] [CrossRef] [Green Version]
















| Proximate Analysis (wt%) | ||
|---|---|---|
| Biomass | Original Char | |
| Moisture | 7.2 | 0.6 |
| Volatile Matter (db) b | 92.8 | 4.53 |
| Ash (db) a | 0.32 | 6.9 |
| Fixed carbon (db) b | 6.88 | 88.57 |
| Ultimate Analysis (wt% db) | ||
| C | 50.9 | 90.6 |
| H | 6.2 | 0.2 |
| N | <0.1 | 0.43 |
| O b | 42.6 | 1.87 |
| Mineral Matter (mg/kg db) | ||
| Na | 111 | 755 |
| K | 340 | 5690 |
| Mg | 114 | 2660 |
| Ca | 562 | 11,200 |
| Fe | 45.5 | 720 |
| Al | 79 | 336 |
| Mn | 69.9 | 1540 |
| P | 37.8 | 677 |
| Si | 43.1 | 1450 |
| 700 °C | 750 °C | 800 °C | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conversions (%) | 0 | 27 | 64 | 72 | 100 | 0 | 27 | 53 | 73 | 91 | 100 | 0 | 25 | 45 | 70 | 85 | 100 | |
| Sample | OC | |||||||||||||||||
| Tests | ||||||||||||||||||
| TGA | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| BET | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| SEM/EDS | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| Samples | 2LC and 48LC | |||||||||||||||||
| Tests | ||||||||||||||||||
| TGA | √ | √ | √ | |||||||||||||||
| BET | √ | √ | √ | |||||||||||||||
| SEM/EDS | √ | √ | √ | |||||||||||||||
| (dX/dt)/Stotal (g/m2-min) | ||||
|---|---|---|---|---|
| Samples | OC | 2LC | 48LC | |
| T (°C) | ||||
| 700 | 5.26 × 10−6 | 7.01 × 10−6 | 5.49 × 10−6 | |
| 750 | 1.45 × 10−5 | 1.90 × 10−5 | 1.49 × 10−5 | |
| 800 | 3.30 × 10−5 | 3.81 × 10−5 | 3.18 × 10−5 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Kantarelis, E.; Engvall, K. Effects of Porous Structure Development and Ash on the Steam Gasification Reactivity of Biochar Residues from a Commercial Gasifier at Different Temperatures. Energies 2020, 13, 5004. https://0-doi-org.brum.beds.ac.uk/10.3390/en13195004
Ding S, Kantarelis E, Engvall K. Effects of Porous Structure Development and Ash on the Steam Gasification Reactivity of Biochar Residues from a Commercial Gasifier at Different Temperatures. Energies. 2020; 13(19):5004. https://0-doi-org.brum.beds.ac.uk/10.3390/en13195004
Chicago/Turabian StyleDing, Saiman, Efthymios Kantarelis, and Klas Engvall. 2020. "Effects of Porous Structure Development and Ash on the Steam Gasification Reactivity of Biochar Residues from a Commercial Gasifier at Different Temperatures" Energies 13, no. 19: 5004. https://0-doi-org.brum.beds.ac.uk/10.3390/en13195004