Biomass Steam Gasification, High-Temperature Gas Cleaning, and SOFC Model: A Parametric Analysis
Abstract
:1. Introduction
2. Simulation Model
2.1. Assumptions
- Process is steady state and isothermal [35];
- All gases behave ideally;
- Catalyst is defined as conventional solid;
- The tars considered are toluene and benzene;
- The inorganic contaminants assumed are hydrogen sulphide and ammonia; and
- Uniform SOFC operating temperature and pressure.
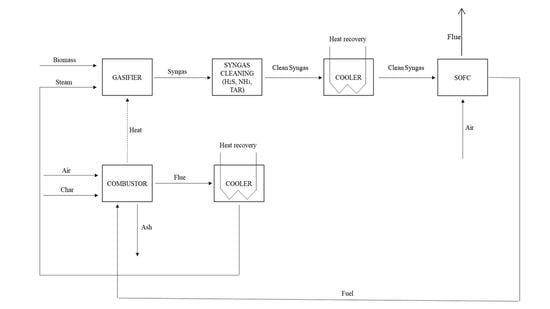
2.2. Process Scheme, Plant, and Model Description
Auxiliary Description
2.3. Gasification Model
2.4. In-Bed Gas Cleaning
2.5. SOFC Model
3. Results and Discussion
3.1. Effect of Steam to Biomass (S/B) Ratio
3.2. Effect of Current Density
3.3. Influence of CO Concentration and Air Flow Rate on SOFC Power Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirmoshtaghi, G.; Skvaril, J.; Campana, P.E.; Li, H.; Thorin, E.; Dahlquist, E. The Influence of different parameters on biomass gasification in circulating fluidized bed gasifiers. Energy Convers. Manag. 2016, 126, 110–123. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, N.; Ding, Y.; Chang, C.; Yuan, T. Thermoeconomic analysis of an integrated combined cooling heating and power system with biomass gasification. Energy Convers. Manag. 2018, 171, 671–682. [Google Scholar] [CrossRef]
- Lauri, P.; Havlík, P.; Kindermann, G.; Forsell, N.; Böttcher, H.; Obersteiner, M. Woody Biomass Energy Potential in 2050. Energy Policy 2014, 66, 19–31. [Google Scholar] [CrossRef]
- Boehman, A.L.; Le Corre, O. Combustion of syngas in internal combustion engines. Combust. Sci. Technol. 2008, 180, 1193–1206. [Google Scholar] [CrossRef]
- Gholamian, E.; Zare, V. A comparative thermodynamic investigation with environmental analysis of SOFC waste heat to power conversion employing Kalina and organic rankine cycles. Energy Convers. Manag. 2016, 117, 150–161. [Google Scholar] [CrossRef]
- Di Carlo, A.; Borello, D.; Bocci, E. Process simulation of a hybrid SOFC/mGT and enriched air/steam fluidized bed gasifier power plant. Int. J. Hydrogen Energy 2013, 38, 5857–5874. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Fragiacomo, P. Energy analysis of an SOFC system fed by syngas. Energy Convers. Manag. 2015, 93, 175–186. [Google Scholar] [CrossRef]
- Lan, W.; Chen, G.; Zhu, X.; Wang, X.; Liu, C.; Xu, B. Biomass gasification-gas turbine combustion for power generation system model based on ASPEN PLUS. Sci. Total. Environ. 2018, 628–629, 1278–1286. [Google Scholar] [CrossRef]
- Villarini, M.; Marcantonio, V.; Colantoni, A.; Bocci, E. Sensitivity analysis of different parameters on the performance of a CHP internal combustion engine system fed by a biomass waste gasifier. Energies 2019, 12, 688. [Google Scholar] [CrossRef] [Green Version]
- Barelli, L.; Bidini, G.; Cinti, G.; Ottaviano, A. SOFC regulation at constant temperature: Experimental test and data regression study. Energy Convers. Manag. 2016, 117, 289–296. [Google Scholar] [CrossRef]
- Kakaç, S.; Pramuanjaroenkij, A.; Yang, X. A review of numerical modeling of solid oxide fuel. Cells 2007, 32, 761–786. [Google Scholar]
- Chiodo, V.; Galvagno, A.; Lanzini, A.; Papurello, D.; Urbani, F.; Santarelli, M.; Freni, S. Biogas reforming process investigation for SOFC application. Energy Convers. Manag. 2015, 98, 252–258. [Google Scholar] [CrossRef]
- Eisavi, B.; Chitsaz, A.; Hosseinpour, J.; Ranjbar, F. Thermo-environmental and economic comparison of three different arrangements of solid oxide fuel cell-gas turbine (SOFC-GT) hybrid systems. Energy Convers. Manag. 2018, 168, 343–356. [Google Scholar] [CrossRef]
- Kuchonthara, P.; Bhattacharya, S.; Tsutsumi, A. Combination of thermochemical recuperative coal gasification cycle and fuel cell for power generation. Fuel 2005, 84, 1019–1021. [Google Scholar] [CrossRef]
- Fung, A.S. Performance of biogas fueled hybrid solid oxide fuel cell (SOFC) and gas turbine cycle. ASME 2018, 2, 1–11. [Google Scholar]
- Hauck, M.; Herrmann, S.; Spliethoff, H. science direct simulation of a reversible SOFC with Aspen Plus. Int. J. Hydrogen Energy 2017, 42, 10329–10340. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Zhao, Y.; Leach, M.; Brandon, N.P.; Shah, N. energy integration and analysis of solid oxide fuel cell based microcombined heat and power systems and other renewable systems using biomass waste derived syngas. Ind. Eng. Chem. Res. 2010, 49, 11506–11516. [Google Scholar] [CrossRef] [Green Version]
- Riensche, E.; Achenbach, E.; Froning, D.; Haines, M.; Heidug, W.; Lokurlu, A.; von Andrian, S. Clean combined-cycle SOFC power plant—Cell modelling and process analysis. J. Power Sources 2000, 86, 404–410. [Google Scholar] [CrossRef]
- Palsson, J.; Selimovic, A.; Sjunnesson, L. Combined solid oxide fuel cell and gas turbine systems for efficient power and heat generation. J. Power Sources 2000, 86, 442–448. [Google Scholar] [CrossRef]
- Fuller, T.A.; Chaney, L.J.; Wolf, T.L.; Kesseli, J.; Nash, J.; Hatvigsen, J. A novel cell/microturbine combined-cycle system. J. Power Sources 2000, 86, 404–410. [Google Scholar]
- Zhang, W.; Croiset, E.; Douglas, P.; Fowler, M.; Entchev, E. Simulation of a tubular solid oxide fuel cell stack using AspenPlusTM unit operation models. Energy Convers. Manag. 2005, 46, 181–196. [Google Scholar] [CrossRef]
- Doherty, W.; Reynolds, A.; Kennedy, D. Computer simulation of a biomass gasi fi cation-solid oxide fuel cell power system using Aspen Plus. Energy 2010, 35, 4545–4555. [Google Scholar] [CrossRef] [Green Version]
- Barisano, D.; Canneto, G.; Rep, M. Deliverable 5.2: UNIfHY 1000 Long Term Tests and Further Hardware Modifications; University of Leeds: Woodhouse, UK, 2018. [Google Scholar]
- Moneti, M.; Di Carlo, A.; Bocci, E.; Foscolo, P.; Villarini, M.; Carlini, M. Influence of the main gasifier parameters on a real system for hydrogen production from biomass. Int. J. Hydrogen Energy 2016, 41, 11965–11973. [Google Scholar] [CrossRef]
- Li, X.; Grace, J.; Watkinson, A.; Lim, C.; Ergüdenler, A. Equilibrium modeling of gasification: A free energy minimization approach and its application to a circulating fluidized bed coal gasifier. Fuel 2001, 80, 195–207. [Google Scholar] [CrossRef]
- Baruah, D.; Baruah, D.C. Modeling of biomass gasification: A review. Renew. Sustain. Energy Rev. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Loha, C.; Chatterjee, P.K.; Chattopadhyay, H. Performance of fluidized bed steam gasification of biomass–Modeling and experiment. Energy Convers. Manag. 2011, 52, 1583–1588. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, W.; Jin, B.; Shao, Y.; Liu, H. Simulation on gasification of forestry residues in fluidized beds by Eulerian–Lagrangian approach. Biores. Technol. 2012, 121, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.; Seo, M.W.; Lim, Y.-I.; Song, B.; Kim, S.-D. CFD simulation with experiments in a dual circulating fluidized bed gasifier. Comput. Chem. Eng. 2012, 36, 48–56. [Google Scholar] [CrossRef]
- Gungor, A. Modeling the effects of the operational parameters on H2 composition in a biomass fluidized bed gasifier. Int. J. Hydrogen Energy 2011, 36, 6592–6600. [Google Scholar] [CrossRef]
- Gumz, W. Gas Producers and Blast Furnaces; University of Michigan: Ann Arbor, MI, USA, 1950. [Google Scholar]
- Marcantonio, V.; Bocci, E.; Monarca, D. Development of a chemical quasi-equilibrium model of biomass waste gasification in a fluidized-bed reactor by using Aspen Plus. Energies 2019, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Olivares, A.; Aznar, M.P.; Caballero, M.A.; Gil, J.; Frances, A.E.; Corella, J. Biomass gasification: Produced gas upgrading by in-bed use of dolomite. Ind. Eng. Chem. Res. 1997, 36, 5220–5226. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Ouweltjes, J.P.; Del Zotto, L.; Monarca, D. Evaluation of sorbents for high temperature removal of tars, hydrogen sulphide, hydrogen chloride and ammonia from biomass-derived syngas by using Aspen Plus. Int. J. Hydrogen Energy 2020, 45, 6651–6662. [Google Scholar] [CrossRef]
- Ye, G.; Xie, N.; Qiao, W.; Grace, J.R.; Lim, C.J. Modeling of fluidized bed membrane reactors for hydrogen production from steam methane reforming with Aspen Plus. Int. J. Hydrogen Energy 2009, 34, 4755–4762. [Google Scholar] [CrossRef]
- Demirbaş, A. Carbonization ranking of selected biomass for charcoal, liquid and gaseous products. Energy Convers. Manag. 2001, 42, 1229–1238. [Google Scholar] [CrossRef]
- Torres, W.; Pansare, S.S.; Goodwin, J.G. Hot gas removal of tars, ammonia, and hydrogen sulfide from biomass gasification gas. Catal. Rev. 2007, 49, 407–456. [Google Scholar] [CrossRef]
- Rapagnà, S.; Orazio, A.D.; Gallucci, K.; Ugo, P. Hydrogen rich gas from catalytic steam gasification of biomass in a fluidized bed containing catalytic filters. Chem. Eng. Trans. 2004, 37, 157–162. [Google Scholar]
- Di Carlo, A.; Borello, D.; Sisinni, M.; Savuto, E.; Venturini, P.; Bocci, E.; Kuramoto, K. Reforming of tar contained in a raw fuel gas from biomass gasification using nickel-mayenite catalyst. Int. J. Hydrogen Energy 2015, 40, 9088–9095. [Google Scholar] [CrossRef]
- Jang, D.H.; Kim, H.T.; Lee, C.; Kim, S.H.; Doherty, W.; Reynolds, A.; Kennedy, D.; Kong, X.; Zhong, W.; Du, W.; et al. Gasification of hazelnut shells in a downdraft gasifier. Energy 2002, 27, 415–427. [Google Scholar]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The study of reactions influencing the biomass steam gasification process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Doherty, W.; Reynolds, A.; Kennedy, D. The effect of air preheating in a biomass CFB gasifier using ASPEN Plus simulation. Biomass Bioenergy 2009, 33, 1158–1167. [Google Scholar] [CrossRef] [Green Version]
- Marcantonio, V.; De Falco, M.; Capocelli, M.; Bocci, E.; Colantoni, A.; Villarini, M. Process analysis of hydrogen production from biomass gasification in fluidized bed reactor with different separation systems. Int. J. Hydrogen Energy 2019, 44, 10350–10360. [Google Scholar]
- Di Carlo, A.; Bocci, E.; Naso, V. Process simulation of a SOFC and double bubbling fluidized bed gasifier power plant. Int. J. Hydrogen Energy 2013, 38, 532–542. [Google Scholar]
- Florin, N.H.; Harris, A.T. Hydrogen production from biomass coupled with carbon dioxide capture: The implications of thermodynamic equilibrium. Int. J. Hydrogen Energy 2007, 32, 4119–4134. [Google Scholar] [CrossRef]
- Inayat, A.; Ahmad, M.M.; Yusup, S.; Mutalib, M.I.A.; Inayat, A.; Ahmad, M.M.; Yusup, S.; Mutalib, M.I.A. Biomass steam gasification with in-situ CO2 capture for enriched hydrogen gas production: A reaction kinetics modelling approach. Energies 2010, 3, 1472–1484. [Google Scholar] [CrossRef] [Green Version]
- Doherty, W.; Reynolds, A.; Kennedy, D. Simulation of a tubular solid oxide fuel cell stack operating on biomass syngas using Aspen Plus. J. Electrochem. Soc. 2010, 157, 5–11. [Google Scholar] [CrossRef] [Green Version]










| ASPEN Plus Name | Block ID | Description |
|---|---|---|
| RYIELD | DECOMP | RYield reactor—converts the non-conventional stream “BIOMASS” into its conventional components |
| RSTOIC | RSTOIC | RStoic reactor—replicates ammonia and hydrogen sulphide |
| CANDLE | RStoic reactor—represents the catalyst filter reaction | |
| AFTERBUR | RStoic reactor—replicates the 100% combustion of hydrogen and carbon monoxide | |
| TARPROD | RStoic reactor—represents the production of C7H8 and C6H6 | |
| COMBUST | RStoic reactor—replicates the combustion of char | |
| SEP | SEP | Separator—divides the biomass in three streams: volatile, char and a stream of NH3 and H2S |
| SEPH2 | Separator—divides a certain part of hydrogen used to produce tar | |
| CATHODE | Separator—replicates the cathode of the SOFC | |
| SEPZNS | Separator—separates the ZNS solid fraction | |
| MIXER | MIX1 | Mixer—mixes oxidising fluid with combustible fluid |
| MIX2 | Mixer—unify the product from gasifier with ammonia and hydrogen sulphide | |
| MIX3 | Mixer—unify the stream S3 and H2 | |
| MIX 4 | Mixer—unify the product from GASIF with tar | |
| FSPLIT | SPLIT | Splitter—divides char unreacted (S3) from char to burn (S4) |
| SPLIT 2 | Splitter—divides char to gasifier and to combustor | |
| SPLIT 3 | Splitter—splits fuel to afterburner and to combustor | |
| RGIBBS | GASIF | Gibbs free energy reactor—represents the gasification zone |
| DOLOMITH | Gibbs free energy reactor—simulates the high temperature reaction od dolomite with NH3, H2S and tar | |
| REQUIL | ZNOZNS | REquil reactor—simulates the reaction of ZnO with H2S |
| ANODE | REquil reactor—simulates the anode of the SOFC | |
| HeatX | EX01 | Heatx—heats up the air to combustor |
| EX02 | Heatx—cools the stream out of candle and heats the one out of ZNOZNS | |
| EX04 | Heatx—preheats the incoming air to cathode and cools the stream from afterburner | |
| EX05 | Heatx—preheats the water with residual heat of afterburner | |
| EX06 | Heatx—heats the water coming out of the EX05 to steam | |
| Heater | EX03 | Heater—cools the stream in input to ZNOZNS |
| Bulk density (kg/m3) | 319.14 |
| Moisture content (wt%) | 7.90 |
| Proximate analysis (%wt, dry basis) | Ash = 0.77; Volatile Matter = 62.70; Fixed Carbon = 24.04 |
| Ultimate analysis (%wt, dry basis) | C = 49.00; H = 6.03; N = 0.22; O = 42.06; Cl = 0.76; S = 0.67 |
| Heating values (MJ/kgdry) | HHV = 20.20; LHV = 18.85 |
| Reaction | Reaction Name | Reaction Number |
|---|---|---|
| Heterogeneous reaction | ||
| Char partial combustion | (3) | |
| Water-gas | (4) | |
| Homogeneous reactions | ||
| partial combustion | (5) | |
| shift | (6) | |
| Steam-methane reforming | (7) |
| Thermal Power Input of the Gasifier (mbio·HHVbio) | 1 MWth | |
|---|---|---|
| Temperatures | Ambient (biomass, combustion air and water for steam production) | 20 °C |
| Gasifier/gasification zone—product gas | 800 °C | |
| Gasifier—steam in | 400 °C | |
| Gasifier/combustion zone—exhaust gas | 800 °C | |
| Gasifier/combustion zone—combustion air in | 400 °C | |
| Sorbent reactor for sulphur removal | 400 °C | |
| SOFC anode | 910 °C | |
| Afterburner/combustion zone - exhaust gas | 910 °C | |
| S/B ratio | 0.5 | |
| System operating pressure | 1 bar |
| Component | Out of the Gasifier (Stream S8) | Out of the Sorbent Reactor DOLOMITH (Stream S10) | Out of the Candle Filter CANLDE (Stream S12) | Out of the Sulphur Removal ZNOZNS |
|---|---|---|---|---|
| H2 (%wet mole fraction) | 32.1 | 32.3 | 41.8 | 41.8 |
| CO (%wet mole fraction) | 14.8 | 14.9 | 18.3 | 18.3 |
| CO2 (%wet mole fraction) | 13.8 | 13.9 | 12.7 | 12.7 |
| H2O (%wet mole fraction) | 29.5 | 30.0 | 22.3 | 22.3 |
| CH4 (%wet mole fraction) | 4.2 | 4.3 | 0.4 | 0.4 |
| NH3 (ppm) | 1719.0 | 86.4 | 78.9 | 75.0 |
| H2S (ppm) | 2287.0 | 345.0 | 315.0 | 0.8 |
| C7H8 (g/Nm3) | 13.1 | 2.6 | 0.02 | 0.02 |
| C6H6 (g/Nm3) | 12.0 | 5.3 | 0.03 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcantonio, V.; Monarca, D.; Villarini, M.; Di Carlo, A.; Del Zotto, L.; Bocci, E. Biomass Steam Gasification, High-Temperature Gas Cleaning, and SOFC Model: A Parametric Analysis. Energies 2020, 13, 5936. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225936
Marcantonio V, Monarca D, Villarini M, Di Carlo A, Del Zotto L, Bocci E. Biomass Steam Gasification, High-Temperature Gas Cleaning, and SOFC Model: A Parametric Analysis. Energies. 2020; 13(22):5936. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225936
Chicago/Turabian StyleMarcantonio, Vera, Danilo Monarca, Mauro Villarini, Andrea Di Carlo, Luca Del Zotto, and Enrico Bocci. 2020. "Biomass Steam Gasification, High-Temperature Gas Cleaning, and SOFC Model: A Parametric Analysis" Energies 13, no. 22: 5936. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225936