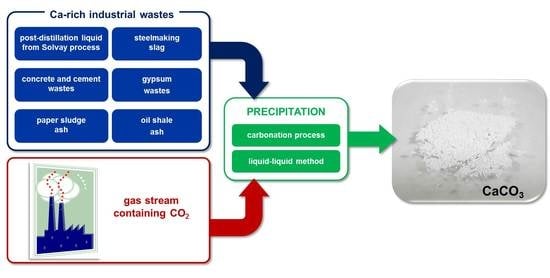
Utilization of Gaseous Carbon Dioxide and Industrial Ca-Rich Waste for Calcium Carbonate Precipitation: A Review
Abstract
:1. Introduction
2. CaCO3 Precipitation Methods
2.1. Carbonation Method
2.2. Liquid-Liquid Method
3. Calcium-Rich Wastes
3.1. Post-Distillation Liquid from Solvay Process
3.1.1. Precipitation Using Aqueous Carbonate Solution
3.1.2. Precipitation Using Flue Gas
3.2. Steelmaking Slag
3.3. Concrete Wastes
3.4. Gypsum Wastes
3.5. Other Ca-Rich Waste
4. Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Zdeb, J.; Howaniec, N.; Smoliński, A. Utilization of carbon dioxide in coal gasification—An experimental study. Energies 2019, 12, 140. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Loo, L.; Konist, A.; Neshumayev, D.; Pihu, T.; Maaten, B.; Siirde, A. Ash and flue gas from oil shale oxy-fuel circulating fluidized bed combustion. Energies 2018, 11, 1218. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.; Kim, S.; Lee, S.; Choi, S.; Kim, M.; Park, Y. Calcium carbonate precipitation for CO2 storage and utilization: A review of the carbonate crystallization and polymorphism. Front. Energy Res. 2017, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Blomen, E.; Hendriks, C.; Neele, F. Capture technologies: Improvements and promising developments. Energy Procedia 2009, 1, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Robles, J.O.; Almaraz, S.D.-L.; Azzaro-Pantel, C. Hydrogen Supply Chain Design: Key Technological Components and Sustainable Assessment. In Hydrogen Supply Chains. Design, Deployment and Operation; Azzaro-Pantel, C., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–79. [Google Scholar]
- COP25 Paris Agreement, European Commission. Available online: https://unfccc.int/cop25 (accessed on 16 November 2020).
- Thambimuthu, K.; Soltanieh, M.; Abanades, J.C. Capture of CO2. In Carbon Dioxide Capture and Storage; Metz, B., Davidson, O., de Coninck, H., Loos, M., Meyer, L., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 105–178. [Google Scholar]
- Plaza, M.G.; Martínez, S.; Rubiera, F. CO2 Capture, Use, and Storage in the Cement Industry: State of the Art and Expectations. Energies 2020, 13, 5692. [Google Scholar] [CrossRef]
- Pellegrini, G.; Strube, R.; Manfrida, G. Comparative study of chemical absorbents in postcombustion CO2 capture. Energy 2010, 35, 851–857. [Google Scholar] [CrossRef]
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Khoo, H.H.; Sharratt, P.N.; Bu, J.; Yeo, T.Y.; Borgna, A.; Highfield, J.G.; Björklöf, T.G.; Zevenhoven, R. Carbon capture and mineralization in singapore: Preliminary environmental impacts and costs via LCA. Ind. Eng. Chem. Res. 2011, 50, 11350–11357. [Google Scholar] [CrossRef]
- Sha, F.; Zhu, N.; Bai, Y.; Li, Q.; Guo, B.; Zhao, T.; Zhang, F.; Zhang, J. Controllable Synthesis of Various CaCO3 Morphologies Based on a CCUS Idea. ACS Sustain. Chem. Eng. 2016, 4, 3032–3044. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Legendre, D.; Said, A.; Järvinen, M. Carbon dioxide dissolution and ammonia losses in bubble columns for precipitated calcium carbonate (PCC) production. Energy 2019, 175, 1121–1129. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef] [Green Version]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. CO2 capture by aqueous Na2CO3 integrated with high-quality CaCO3 formation and pure CO2 release at room conditions. J. CO2 Util. 2017, 22, 346–354. [Google Scholar] [CrossRef]
- Abidin, V.; Bouallou, C.; Clodic, D. Valorization of CO2 emissions into ethanol by an innovative process. Chem. Eng. Trans. 2011, 25, 1–6. [Google Scholar] [CrossRef]
- Mendes, L.; De Medeiros, J.L.; Alves, R.M.B.; Araújo, O.Q.F. Production of methanol and organic carbonates for chemical sequestration of CO2 from an NGCC power plant. Clean Technol. Environ. Policy 2014, 16, 1095–1105. [Google Scholar] [CrossRef]
- Woodall, C.M.; McQueen, N.; Pilorgé, H.; Wilcox, J. Utilization of mineral carbonation products: Current state and potential. Greenh. Gases Sci. Technol. 2019, 9, 1096–1113. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Liu, Y.; Ren, Y.; Yan, H.; Wang, M.; Wang, D.; Lu, X.Y.; Wang, B.; Fan, T.; Guo, H. Controllable synthesis of all the anhydrous CaCO3 polymorphs with various morphologies in CaCl2-NH3-CO2 aqueous system. Powder Technol. 2018, 333, 410–420. [Google Scholar] [CrossRef]
- Ding, L.; Wu, B.; Luo, P. Preparation of CaCO3 nanoparticles in a surface-aerated tank stirred by a long-short blades agitator. Powder Technol. 2018, 333, 339–346. [Google Scholar] [CrossRef]
- Wachi, S.; Jones, A.G. Mass Transfer with Chemical Precipitation Reaction. Chem. Eng. Sci. 1991, 46, 1027–1033. [Google Scholar] [CrossRef]
- Green, D.W.; Perry, R.H. (Eds.) Perry’s Chemical Engineers’ Handbook, 8th ed.; The McGraw-Hill Companies: New York, NY, USA, 2008. [Google Scholar]
- Mori, Y.; Enomae, T.; Isogai, A. Preparation of pure vaterite by simple mechanical mixing of two aqueous salt solutions. Mater. Sci. Eng. C 2009, 29, 1409–1414. [Google Scholar] [CrossRef]
- Ukrainczyk, M.; Kontrec, J.; Babić-Ivančić, V.; Brečević, L.; Kralj, D. Experimental design approach to calcium carbonate precipitation in a semicontinuous process. Powder Technol. 2007, 171, 192–199. [Google Scholar] [CrossRef]
- Kitamura, M. Controlling factor of polymorphism in crystallization process. J. Cryst. Growth 2002, 237–239, 2205–2214. [Google Scholar] [CrossRef]
- Kralj, D.; Kontrec, J.; Brečević, L.; Falini, G.; Nöthig-Laslo, V. Effect of Inorganic Anions on the Morphology and Structure of Magnesium Calcite. Chem. A Eur. J. 2004, 10, 1647–1656. [Google Scholar] [CrossRef]
- Wen, Y.; Xiang, L.; Jin, Y. Synthesis of plate-like calcium carbonate via carbonation route. Mater. Lett. 2003, 57, 2565–2571. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Mohammad, A.F.; Suleiman, M.I.; Al Musharfy, M.; Al-Marzouqi, A.H. A new process for the capture of CO2 and reduction of water salinity. Desalination 2017, 411, 69–75. [Google Scholar] [CrossRef]
- Kasikowski, T.; Buczkowski, R.; Lemanowska, E. Cleaner production in the ammonia-soda industry: An ecological and economic study. J. Environ. Manag. 2004, 73, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, G. Cleaner production in the Solvay Process: General strategies and recent developments. J. Clean. Prod. 2008, 16, 833–841. [Google Scholar] [CrossRef]
- Hulisz, P.; Piernik, A. Soils affected by soda industry in Inowrocław. In Technologenic Soils of Poland; Charzyński, P., Hulisz, P., Bednarek, R., Eds.; Polish Society of Soil Science: Toruń, Poland, 2013; pp. 125–140. [Google Scholar]
- Somani, R.S.; Patel, K.S.; Mehta, A.R.; Jasra, R.V. Examination of the polymorphs and particle size of calcium carbonate precipitated using still effluent (i.e., CaCl2 + NaCl solution) of soda ash manufacturing process. Ind. Eng. Chem. Res. 2006, 45, 5223–5230. [Google Scholar] [CrossRef]
- Białowicz, K.; Kiełkowska, U. Precipitation of calcium carbonate in the presence of urea at 293K and 343K. Polish J. Chem. Technol. 2014, 16, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Trypuć, M.; Białowicz, K. CaCO3 production using liquid waste from Solvay method. J. Clean. Prod. 2011, 19, 751–756. [Google Scholar] [CrossRef]
- Gao, C.; Dong, Y.; Zhang, H.; Zhang, J. Utilization of distiller waste and residual mother liquor to prepare precipitated calcium carbonate. J. Clean. Prod. 2007, 15, 1419–1425. [Google Scholar] [CrossRef]
- Mikhailova, E.; Panasenko, V.; Markova, N. Calcium carbonate synthesis with prescribed properties based on liquid waste of soda production. Odes’kyi Politech. Universytet Pr. 2016, 2, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Alamdari, A.; Alamdari, A.; Mowla, D. Kinetics of calcium carbonate precipitation through CO2 absorption from flue gas into distiller waste of soda ash plant. J. Ind. Eng. Chem. 2014, 20, 3480–3486. [Google Scholar] [CrossRef]
- Czaplicka, N.; Konopacka-Łyskawa, D.; Kościelska, B.; Łapinski, M. Effect of selected ammonia escape inhibitors on carbon dioxide capture and utilization via calcium carbonate precipitation. J. CO2 Util. 2020, 42. [Google Scholar] [CrossRef]
- Czaplicka, N.; Konopacka-Łyskawa, D. Studies on the utilization of post-distillation liquid from Solvay process to carbon dioxide capture and storage. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef] [Green Version]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Kuzharov, A.S.; Lipkin, M.S.; Kuzharov, A.A.; Lipkin, V.M.; Nguen, K.; Shishka, V.G.; Rybalko, E.A.; Lytkin, N.A.; Misharev, A.S.; Tulaeva, F.R.; et al. Green tribology: Disposal and recycling of waste Ni–Cd batteries to produce functional tribological materials. J. Frict. Wear 2015, 36, 306–313. [Google Scholar] [CrossRef]
- Hall, C.; Large, D.J.; Adderley, B.; West, H.M. Calcium leaching from waste steelmaking slag: Significance of leachate chemistry and effects on slag grain mineralogy. Miner. Eng. 2014, 65, 156–162. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, M.S.; Zhang, J.P.; Yang, G. Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem. Eng. J. 2011, 173, 437–445. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Chen, G.; Sun, Z.; Xu, Y.; Yu, J. Preparation of calcium carbonate and hydrogen chloride from distiller waste based on reactive extraction–crystallization process. Chem. Eng. J. 2015, 278, 55–61. [Google Scholar] [CrossRef]
- Dong, C.; Song, X.; Li, Y.; Liu, C.; Chen, H.; Yu, J. Impurity ions effect on CO2 mineralization via coupled reaction-extraction-crystallization process of CaCl2 waste liquids. J. CO2 Util. 2018, 27, 115–128. [Google Scholar] [CrossRef]
- Chen, T.; Neville, A.; Yuan, M. Assessing the effect of Mg2+ CaCO3 scale formation-bulk precipitation and surface deposition. J. Cryst. Growth 2005, 275, 2–8. [Google Scholar] [CrossRef]
- Xu, X.; Guo, H.; Cheng, X.; Li, M. The promotion of magnesium ions on aragonite precipitation in MICP process. Constr. Build. Mater. 2020, 263, 120057. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, Q.; Wei, C.; Lin, X.; Dong, J.; Liao, C.; Xu, A. Closed-circulating CO2 sequestration process evaluation utilizing wastes in steelmaking plant. Sci. Total Environ. 2020, 738, 139747. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Teir, S.; Eloneva, S.; Fogelholm, C.J.; Zevenhoven, R. Dissolution of steelmaking slags in acetic acid for precipitated calcium carbonate production. Energy 2007, 32, 528–539. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M. Mechanical performance and microstructure of the calcium carbonate binders produced by carbonating steel slag paste under CO2 curing. Cem. Concr. Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Eloneva, S.; Teir, S.; Salminen, J.; Fogelholm, C.J.; Zevenhoven, R. Steel converter slag as a raw material for precipitation of pure calcium carbonate. Ind. Eng. Chem. Res. 2008, 47, 7104–7111. [Google Scholar] [CrossRef]
- Kojima, T.; Nagamine, A.; Ueno, N.; Uemiya, S. Absorption and fixation of carbon dioxide by rock weathering. Energy Convers. Manag. 1997, 38, S461–S466. [Google Scholar] [CrossRef]
- Kakizawa, A.M.; Yamasaki, Y.Y. A new CO2 disposal process via artificial weathering of calcium silicate accelerated by acetic acid. Energy 2001, 26, 341–354. [Google Scholar] [CrossRef]
- Eloneva, S.; Said, A.; Fogelholm, C.J.; Zevenhoven, R. Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl. Energy 2012, 90, 329–334. [Google Scholar] [CrossRef]
- Mattila, H.P.; Grigaliu-naite, I.; Zevenhoven, R. Chemical kinetics modeling and process parameter sensitivity for precipitated calcium carbonate production from steelmaking slags. Chem. Eng. J. 2012, 192, 77–89. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Zevenhoven, R. Co-utilization of CO2 and calcium silicate-rich slags for Precipitated calcium carbonate production (Part I). In Proceedings of ECOS 2005, the 18th International Conference on Efficiency, Cost, Optimization, Simulation, and Environmental Impact of Energy Systems, Trondheim, Norway, 20–22 June 2005; Tapir Academic Press: Trondheim, Norway, 2005; Volume 2, pp. 749–756. [Google Scholar]
- Said, A.; Laukkanen, T.; Järvinen, M. Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl. Energy 2016, 177, 602–611. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Jeong, S.K.; Youn, M.H.; Nguyen, D.D.; Chang, S.W.; Kim, S.S. Calcium extraction from steelmaking slag and production of precipitated calcium carbonate from calcium oxide for carbon dioxide fixation. J. Ind. Eng. Chem. 2017, 53, 233–240. [Google Scholar] [CrossRef]
- Kodama, S.; Nishimoto, T.; Yamamoto, N.; Yogo, K.; Yamada, K. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 2008, 33, 776–784. [Google Scholar] [CrossRef]
- Teir, S.; Kotiranta, T.; Pakarinen, J.; Mattila, H.P. Case study for production of calcium carbonate from carbon dioxide in flue gases and steelmaking slag. J. CO2 Util. 2016, 14, 37–46. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.C. Potential of CO2 sequestration through construction and demolition (C&D) waste—An overview. J. CO2 Util. 2017, 20, 234–242. [Google Scholar] [CrossRef]
- Kang, S.H.; Kwon, Y.H.; Moon, J. Quantitative analysis of CO2 uptake and mechanical properties of air lime-based materials. Energies 2019, 12, 2903. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Ling, T.C.; Mo, K.H. Recycling of wastes for value-added applications in concrete blocks: An overview. Resour. Conserv. Recycl. 2018, 138, 298–312. [Google Scholar] [CrossRef]
- Van der Zee, S.; Zeman, F. Production of carbon negative precipitated calcium carbonate from waste concrete. Can. J. Chem. Eng. 2016, 94, 2153–2159. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Tam, C.M.; Le, K.N. Removal of cement mortar remains from recycled aggregate using pre-soaking approaches. Resour. Conserv. Recycl. 2007, 50, 82–101. [Google Scholar] [CrossRef] [Green Version]
- Vanderzee, S.; Zeman, F. Recovery and carbonation of 100% of calcium in waste concrete fines: Experimental results. J. Clean. Prod. 2018, 174, 718–727. [Google Scholar] [CrossRef]
- Martín, D.; Flores-Alés, V.; Aparicio, P. Proposed Methodology to Evaluate CO2 Capture Using Construction and Demolition Waste. Minerals 2019, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Martín, D.; Aparicio, P.; Galán, E. Accelerated carbonation of ceramic materials. Application to bricks from Andalusian factories (Spain). Constr. Build. Mater. 2018, 181, 598–608. [Google Scholar] [CrossRef]
- Ben Ghacham, A.; Cecchi, E.; Pasquier, L.C.; Blais, J.F.; Mercier, G. CO2 sequestration using waste concrete and anorthosite tailings by direct mineral carbonation in gas-solid-liquid and gas-solid routes. J. Environ. Manag. 2015, 163, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Shuto, D.; Igarashi, K.; Nagasawa, H.; Iizuka, A.; Inoue, M.; Noguchi, M.; Yamasaki, A. CO2 Fixation Process with Waste Cement Powder via Regeneration of Alkali and Acid by Electrodialysis: Effect of Operation Conditions. Ind. Eng. Chem. Res. 2015, 54, 6569–6577. [Google Scholar] [CrossRef]
- Jo, H.; Park, S.H.; Jang, Y.N.; Chae, S.C.; Lee, P.K.; Jo, H.Y. Metal extraction and indirect mineral carbonation of waste cement material using ammonium salt solutions. Chem. Eng. J. 2014, 254, 313–323. [Google Scholar] [CrossRef]
- Shuto, D.; Nagasawa, H.; Iizuka, A.; Yamasaki, A. A CO2 fixation process with waste cement powder via regeneration of alkali and acid by electrodialysis. RSC Adv. 2014, 4, 19778–19788. [Google Scholar] [CrossRef]
- Arce, G.L.A.F.; Okamoto, S.; Dos Santos, J.C.; de Carvalho, J.A.; Avila, I.; Romero Luna, C.M.; Gomes Soares Neto, T. CO2 sequestration by pH-swing mineral carbonation based on HCl/NH4OH system using iron-rich lizardite 1T. J. CO2 Util. 2018, 24, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Arce, G.L.A.F.; Soares Neto, T.G.; Ávila, I.; Luna, C.M.R.; Carvalho, J.A. Leaching optimization of mining wastes with lizardite and brucite contents for use in indirect mineral carbonation through the pH swing method. J. Clean. Prod. 2017, 141, 1324–1336. [Google Scholar] [CrossRef] [Green Version]
- Kunzler, C.; Alves, N.; Pereira, E.; Nienczewski, J.; Ligabue, R.; Einloft, S.; Dullius, J. CO2 storage with indirect carbonation using industrial waste. Energy Procedia 2011, 4, 1010–1017. [Google Scholar] [CrossRef] [Green Version]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Junin, R.; Hamidi, H.; Manan, M.; Daud, A.R.M. Mineral carbonation of red gypsum via pH-swing process: Effect of CO2 pressure on the efficiency and products characteristics. Chem. Eng. J. 2015, 264, 425–436. [Google Scholar] [CrossRef]
- Jo, H.; Jo, H.Y.; Jang, Y.N. Effect of extraction solutions on carbonation of cementitious materials in aqueous solutions. Environ. Technol. 2012, 33, 1391–1401. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.C.; Mo, K.H. Valorization of waste powders from cement-concrete life cycle: A pathway to circular future. J. Clean. Prod. 2020, 268, 122358. [Google Scholar] [CrossRef]
- Iizuka, A.; Sasaki, T.; Honma, M.; Yoshida, H.; Hayakawa, Y.; Yanagisawa, Y.; Yamasaki, A. Pilot-Scale Operation of a Concrete Sludge Recycling Plant and Simultaneous Production of Calcium Carbonate. Chem. Eng. Commun. 2017, 204, 79–85. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Guimaraes, M.; Nilsson, A. The CO2 Balance of Concrete in a Life Cycle Perspective; Danish Technological-DTI: Taastrup, Denmark, 2005; ISBN 8777567587. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.533.3817&rep=rep1&type=pdf (accessed on 10 August 2020).
- Zhang, Y.; Wang, R.; Liu, Z.; Zhang, Z. A novel carbonate binder from waste hydrated cement paste for utilization of CO2. J. CO2 Util. 2019, 32, 276–280. [Google Scholar] [CrossRef]
- Iizuka, A.; Sakai, Y.; Yamasaki, A.; Honma, M.; Hayakawa, Y.; Yanagisawa, Y. Bench-scale operation of a concrete sludge recycling plant. Ind. Eng. Chem. Res. 2012, 51, 6099–6104. [Google Scholar] [CrossRef]
- Iizuka, A.; Fujii, M.; Yamasaki, A.; Yanagisawa, Y. Development of a new CO2 sequestration process utilizing the carbonation of waste cement. Ind. Eng. Chem. Res. 2004, 43, 7880–7887. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Yamasaki, A.; Iizuka, A.; Fujii, M.; Kumagai, K.; Yanagisawa, Y. Development of a process for producing high-purity calcium carbonate (CaCO3) from waste cement using pressurized CO2. Environ. Prog. 2005, 24, 162–170. [Google Scholar] [CrossRef]
- Altiner, M.; Top, S.; Kaymakoǧlu, B.; Seçkin, I.Y.; Vapur, H. Production of precipitated calcium carbonate particles from gypsum waste using venturi tubes as a carbonation zone. J. CO2 Util. 2019, 29, 117–125. [Google Scholar] [CrossRef]
- Msila, X.; Billing, D.G.; Barnard, W. Capture and storage of CO2 into waste phosphogypsum: The modified Merseburg process. Clean Technol. Environ. Policy 2016, 18, 2709–2715. [Google Scholar] [CrossRef]
- Song, K.; Jang, Y.N.; Kim, W.; Lee, M.G.; Shin, D.; Bang, J.H.; Jeon, C.W.; Chae, S.C. Precipitation of calcium carbonate during direct aqueous carbonation of flue gas desulfurization gypsum. Chem. Eng. J. 2012, 213, 251–258. [Google Scholar] [CrossRef]
- Song, K.; Kim, W.; Bang, J.H.; Park, S.; Jeon, C.W. Polymorphs of pure calcium carbonate prepared by the mineral carbonation of flue gas desulfurization gypsum. Mater. Des. 2015, 83, 308–313. [Google Scholar] [CrossRef]
- Lu, S.Q.; Lan, P.Q.; Wu, S.F. Preparation of Nano-CaCO3 from Phosphogypsum by Gas-Liquid-Solid Reaction for CO2 Sorption. Ind. Eng. Chem. Res. 2016, 55, 10172–10177. [Google Scholar] [CrossRef]
- Spathi, C.; Young, N.; Heng, J.Y.Y.; Vandeperre, L.J.M.; Cheeseman, C.R. A simple method for preparing super-hydrophobic powder from paper sludge ash. Mater. Lett. 2015, 142, 80–83. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Wiklund, A.; Fagerlund, J.; Eloneva, S.; Veen, B.I.; Geerlings, H.; Van Mossel, G.; Boerrigter, H. Carbonation of calcium-containing mineral and industrial by-products. Front. Chem. Eng. China 2010, 4, 110–119. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, D. Maximization of CO2 storage for various solvent types in indirect carbonation using paper sludge ash. Environ. Sci. Pollut. Res. 2018, 25, 30101–30109. [Google Scholar] [CrossRef]
- Uibu, M.; Velts, O.; Kuusik, R. Developments in CO2 mineral carbonation of oil shale ash. J. Hazard. Mater. 2010, 174, 209–214. [Google Scholar] [CrossRef]
- Velts, O.; Uibu, M.; Kallas, J.; Kuusik, R. Waste oil shale ash as a novel source of calcium for precipitated calcium carbonate: Carbonation mechanism, modeling, and product characterization. J. Hazard. Mater. 2011, 195, 139–146. [Google Scholar] [CrossRef]
- Velts, O.; Hautaniemi, M.; Kallas, J.; Kuusik, R. Modeling calcium dissolution from oil shale ash: Part 1. Ca dissolution during ash washing in a batch reactor. Fuel Process. Technol. 2010, 91, 486–490. [Google Scholar] [CrossRef]
- Tamm, K.; Kallas, J.; Kuusik, R.; Uibu, M. Modelling Continuous Process for Precipitated Calcium Carbonate Production from Oil Shale Ash. Energy Procedia 2017, 114, 5409–5416. [Google Scholar] [CrossRef]
- Park, S.; Ahn, Y.; Lee, S.; Choi, J. Calcium carbonate synthesis from waste concrete for carbon dioxide capture: From laboratory to pilot scale. J. Hazard. Mater. 2021, 403, 123862. [Google Scholar] [CrossRef]
- Dadsetani, R.; Sheikhzadeh, G.A.; Safaei, M.R.; Alnaqi, A.A.; Amiriyoon, A. Exergoeconomic optimization of liquefying cycle for noble gas argon. Heat Mass Transf. 2019, 55, 1995–2007. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R.; Leon, A.S.; Khaled, U.; Goodarzi, M.; Meer, R. Energetic analysis of different configurations of power plants connected to liquid chemical looping gasification. Processes 2019, 7, 763. [Google Scholar] [CrossRef] [Green Version]
- Mehrdad, S.; Dadsetani, R.; Amiriyoon, A.; Leon, A.S.; Safaei, M.R.; Goodarzi, M. Exergo-economic optimization of organic rankine cycle for saving of thermal energy in a sample power plant by using of strength pareto evolutionary algorithm II. Processes 2020, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Osagie, E.; Aliyu, A.M.; Nnabuife, S.G.; Omoregbe, O.; Etim, V. Exergy analysis and evaluation of the different flowsheeting configurations for CO2 capture plant using 2-amino-2-methyl-1-propanol (AMP). Processes 2019, 7, 391. [Google Scholar] [CrossRef] [Green Version]
- Haghbakhsh, R.; Raeissi, S. Deep eutectic solvents for CO2 capture from natural gas by energy and exergy analyses. J. Environ. Chem. Eng. 2019, 7, 103411. [Google Scholar] [CrossRef]
- Romeo, L.M.; Guedea, I.; Lupiañez, C. Exergetic comparison of different oxyfuel technologies. Int. J. Energy Environ. Eng. 2011, 2, 35–37. Available online: http://ijeee.azad.ac.ir/article_510894.html (accessed on 16 November 2020).
- Mukherjee, S.; Kumar, P.; Yang, A.; Fennell, P. Energy and exergy analysis of chemical looping combustion technology and comparison with pre-combustion and oxy-fuel combustion technologies for CO2 capture. J. Environ. Chem. Eng. 2015, 3, 2104–2114. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Wang, Q.; Guo, J.; Liu, Z.; Zheng, C. Exergy-based control strategy selection for flue gas recycle in oxy-fuel combustion plant. Fuel 2015, 161, 87–96. [Google Scholar] [CrossRef]
- Farooqui, A.; Bose, A.; Ferrero, D.; Llorca, J.; Santarelli, M. Techno-economic and exergetic assessment of an oxy-fuel power plant fueled by syngas produced by chemical looping CO2 and H2O dissociation. J. CO2 Util. 2018, 27, 500–517. [Google Scholar] [CrossRef]
- Lee, B.J.; Lee, J.I.; Yun, S.Y.; Lim, C.S.; Park, Y.K. Economic evaluation of carbon capture and utilization applying the technology of mineral carbonation at coal-fired power plant. Sustainability 2020, 12, 6175. [Google Scholar] [CrossRef]
- Zimmermann, A.W.; Wunderlich, J.; Müller, L.; Buchner, G.A.; Marxen, A.; Michailos, S.; Armstrong, K.; Naims, H.; McCord, S.; Styring, P.; et al. Techno-Economic Assessment Guidelines for CO2 Utilization. Front. Energy Res. 2020, 8, 1–23. [Google Scholar] [CrossRef] [Green Version]







| Ca2+ Source | CO32− Source | Reaction Conditions | Product Characteristic | Ref. |
|---|---|---|---|---|
| Post-distillation liquid (1.25 mol/dm3 CaCl2, 0.85 mol/dm3 NaCl) | Aqueous Na2CO3 solution (0.4–3 mol/dm3) | 30–95 °C Atmospheric pressure |
| [40] |
| Post-distillation liquid from Janikowo Soda Factory, Poland | Aqueous NaHCO3 solution (1.158–1.226 mol/dm3) | 20, 70 °C Atmospheric pressure |
| [41] |
| Post-distillation liquid (CaCl2) | Post-filtration solution ((NH4)2CO3) | 30 °C Atmospheric pressure |
| [42] |
| Post-distillation liquid (15.28% CaCl2, 0.11% NaCl) | Post-filtration solution (7.31% NaCl, 6.43% (NH4)2CO3, 16.58% NH4Cl) | 5–40 °C Atmospheric pressure |
| [43] |
| Post-distillation liquid (1.35 mol/dm3 CaCl2, 1.19 mol/dm3 NaCl) | Mother solution (1.73 mol/dm3 NaHCO3, 0.5 mol/dm3 Na2CO3, 0.09 mol/dm3 NaCl) | 70–90 °C Atmospheric pressure |
| [44] |
| Ca2+ Source | Gas Mixture Composition | Temperature | Pressure | Product Characteristic | Ref. |
|---|---|---|---|---|---|
| Aqueous CaCl2 solution | 15% vol. CO2 in air | 20 °C | Atmospheric |
| [22] |
| Post-distillation liquid | Fumes created during the combustion of 3 g coal samples | 20, 40, 60 °C | Atmospheric |
| [37] |
| Post-distillation liquid (112 g/dm3 CaCl2, 56 g/dm3 NaCl) | 15 and 100% vol. CO2 in air | Room | Atmospheric |
| [47] |
| Aqueous CaCl2 solution (1 mol/dm3) | 100% vol. CO2 | 20 °C | Atmospheric |
| [53] |
| Aqueous CaCl2 solution (0.025–3 mol/dm3) | 100% vol. CO2 | 5–55 °C | Atmospheric |
| [52] |
| Type of Slag | Extraction Agent | Gas Mixture Composition | Temperature | Pressure | Product Characteristic | Ref. |
|---|---|---|---|---|---|---|
| Oxygen furnace steelmaking slag | NH4Cl aqueous solution | n.d. | 40, 60, 80 °C | 10, 20, 30, 40 bar |
| [51] |
| Steel converter slag | Aqueous acetic acid solution (1 M) | 10, 25, 50, 100% vol. CO2 in N2 | 30, 70 °C | Atmospheric |
| [61] |
| Steel converter slag | Aqueous ammonium salt solution (NH4Cl, NH4NO3, CH3COONH4) | 10, 25, 50, 100% vol. CO2 in N2 | 20–70 °C | Atmospheric |
| [65] |
| Steel converter slag | 1 M NH4Cl aqueous solution | n.d. | 20, 45 °C | Atmospheric |
| [67] |
| Steelmaking slag | 1 M NH4Cl aqueous solution | 99.999% vol. CO2 | 25–80 °C | Atmospheric |
| [68] |
| Argon oxygen decarburisation slag | 2 M aqueous ammonium salt solution (NH4Cl, NH4NO3, CH3COONH4) | 22% vol. CO2 | 60 °C | Atmospheric |
| [70] |
| Raw Material | Extraction Agent | Gas Mixture Composition | Temperature | Pressure | Product Characteristic | Ref. |
|---|---|---|---|---|---|---|
| Waste concrete | NH4Cl, HCl, CH3COOH, water | 99.9% vol. of CO2 | Room | Atmospheric |
| [87] |
| Concrete sludge (waste cement) | Water (hydration) | 6–13% vol. of CO2 | Room | Atmospheric |
| [89] |
| Concrete sludge (waste cement) | Water (hydration) | 8 and 13% vol. of CO2 | Room | Atmospheric |
| [92] |
| Concrete sludge (waste cement) | Carbonic acid solution (pressurized CO2) | n.d. | 30, 50, 70 °C | Atmospheric |
| [94] |
| Raw Material | Extraction Agent | Gas Mixture Composition | Temperature | Pressure | Product Characteristic | Ref. |
|---|---|---|---|---|---|---|
| PG | Aqueous NH3 | Pure CO2 | Room | Atmospheric |
| [96] |
| DG | Aqueous NH3 | 99.99% vol. of CO2 | Room | Atmospheric |
| [97] |
| DG | Aqueous NH3 | 99.99% vol. of CO2 | Room | Atmospheric | Regardless of the amount of NH3 in the solution:
Stoichiometric amount of NH3:
Excess of NH3:
| [98] |
| DG | Aqueous NH3 | Pure CO2 | 20, 30, 40 °C | Atmospheric |
| [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaplicka, N.; Konopacka-Łyskawa, D. Utilization of Gaseous Carbon Dioxide and Industrial Ca-Rich Waste for Calcium Carbonate Precipitation: A Review. Energies 2020, 13, 6239. https://0-doi-org.brum.beds.ac.uk/10.3390/en13236239
Czaplicka N, Konopacka-Łyskawa D. Utilization of Gaseous Carbon Dioxide and Industrial Ca-Rich Waste for Calcium Carbonate Precipitation: A Review. Energies. 2020; 13(23):6239. https://0-doi-org.brum.beds.ac.uk/10.3390/en13236239
Chicago/Turabian StyleCzaplicka, Natalia, and Donata Konopacka-Łyskawa. 2020. "Utilization of Gaseous Carbon Dioxide and Industrial Ca-Rich Waste for Calcium Carbonate Precipitation: A Review" Energies 13, no. 23: 6239. https://0-doi-org.brum.beds.ac.uk/10.3390/en13236239