Energy Recovery from Waste Tires Using Pyrolysis: Palestine as Case of Study
Abstract
:1. Introduction
Pyrolysis Technology and Energy Security in Palestine
2. Literature Review
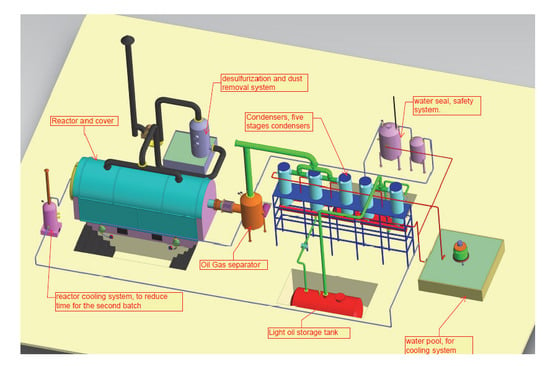
3. Methodology
Analaytical Techniques
4. Results and Discussion
4.1. Yields of the Pyrolysis Products
4.2. Characterization of Solid Tires and Pyrolysis Carbon Black (PCB)
4.3. Characterization of Tire Pyrolysis Oil (TPO)
4.4. Characterization of the Pyrolysis Gas (Pyro-Gas) Fraction
5. Summary
6. Conclusions and Recommendations
- Handling and transportation of the TPO and PCB must be in a closed system.
- To produce alternative diesel from pyrolysis tire oil (TPO), more filtration, desulfurization and wet scrubber with CaOH column must be added.
- To make the technology sustainable, better utilization of PCB must be established.
- The workers in the pyrolysis plant must be skillful and well trained.
- There is chlorine present and there is a risk of the formation of carcinogenic dioxins. However, the objective of this research is the energy recovery from waste tires using pyrolysis and the analysis of risk of the formation of carcinogenic dioxins can be considered in the next research paper.
- Finally, it can be summarized that the pyrolysis plant should be operated only under constant monitoring and advisory support by a team specialized in tire pyrolysis, since it is a new technology.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviation | Definition |
| BR | Polybutadiene |
| NR | Natural Rubber |
| PCB | Pyrolysis Carbon Black |
| Pyro-Gas | Pyrolysis Gas |
| SBR | Styrene Butadiene |
| TPO | Tire Pyrolysis Oil |
| PET | Polyethylene Terephthalate |
| HHV | High Heating Value |
| ASTM | American Society for Testing and Materials |
| TGA | Thermogravimetric Analysis |
| DTG | Derivative Thermogravimetry |
References
- Palestinian Central Bureau of Statistics (PCBS). State of Palestine, Household Energy Survey. Available online: http://www.pcbs.gov.ps/ (accessed on 14 June 2019).
- Huffman, G.P.; Shah, N. Can waste plastics and tires be recycled economically. Chemtech 1998, 28, 34–43. [Google Scholar]
- Wang, H.; Xu, H.; Xuan, X. Review of waste tire reuse and recycling in China –current situation, problems and countermeasures. Adv. Nat. Sci. 2013, 1, 81–90. [Google Scholar]
- Amer EL-Hamouz. The Development of National Mangle Plan for Hazardous Waste Management for Palestinian National Authority. 2010. Available online: http://environment.pna.ps/ar/files/Part_one_Final_Report_on_The_Development_of_a_National_Master_Plan_for_Hazardous_Waste_Management_for_the_Palestinian_National_Authority_en.pdf (accessed on 29 June 2019).
- Islam, M.R.; Islam, M.N.; Mustafi, N.N.; Rahim, M.A.; Haniu, H. Thermal recycling of solid tire wastes for alternative liquid fuel: The first commercial step in Bangladesh. Procedia Eng. 2013, 56, 573–582. [Google Scholar] [CrossRef] [Green Version]
- International Energy Agency. Technology Roadmap: Biofuels for Transport; International Energy Agency: Paris, France, 2011. [Google Scholar] [CrossRef]
- Aydın, H.; İlkılıç, C. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel 2012, 102, 605–612. [Google Scholar] [CrossRef]
- Bekhrad, K.; Aslani, A.; Mazzuca-Sobczuk, T. Energy Security in Andalusia: The Role of Renewable Energy Sources. Case Stud. Chem. Environ. Eng. 2019, 100001. [Google Scholar] [CrossRef]
- Juaidi, A.; Montoya, F.G.; Ibrik, I.H.; Manzano-Agugliaro, F. An overview of renewable energy potential in Palestine. Renew. Sustain. Energy Rev. 2016, 65, 943–960. [Google Scholar] [CrossRef]
- Juaidi, A.; Montoya, F.G.; Gázquez, J.A.; Manzano-Agugliaro, F. An overview of energy balance compared to sustainable energy in United Arab Emirates. Renew. Sustain. Energy Rev. 2016, 55, 1195–1209. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Konda, N.M.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: Review of the state of art and future research directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Azzuni, A.; Breyer, C. Energy security and energy storage technologies. Energy Procedia 2018, 155, 237–258. [Google Scholar] [CrossRef]
- Gillessen, B.; Heinrichs, H.; Hake, J.F.; Allelein, H.J. Energy security in context of transforming energy systems: A case study for natural gas transport in Germany. Energy Procedia 2019, 158, 3339–3345. [Google Scholar] [CrossRef]
- Kramer, C.A.; Loloee, R.; Wichman, I.S.; Ghosh, R.N. Time resolved measurements of pyrolysis products from thermoplastic poly-methyl-methacrylate (PMMA). In ASME 2009 International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers Digital Collection: New York, NY, USA, 2009; pp. 99–105. [Google Scholar]
- Chowdhury, R.; Sarkar, A. Reaction kinetics and product distribution of slow pyrolysis of Indian textile wastes. Int. J. Chem. React. Eng. 2012, 10. [Google Scholar] [CrossRef]
- Biswal, B.; Kumar, S.; Singh, R.K. Production of hydrocarbon liquid by thermal pyrolysis of paper cup waste. J. Waste Manag. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Kagayama, M.; Igarashi, M.; Fukada, J.; Kunii, D. Thermal Conversion of Solid Wastes and Biomass; America Chemical Society: Washington, DC, USA, 1980; Volume 130, pp. 527–531. [Google Scholar]
- Kawakami, S.; Inoue, K.; Tanaka, H.; Sakai, T. Pyrolysis Process for Scrap Tires. In Thermal Conversion of Solid Wastes and Biomass; Chapter 40; ACS Publications: Washington, DC, USA, 1980; pp. 557–572. Available online: https://0-pubs-acs-org.brum.beds.ac.uk/doi/pdf/10.1021/bk-1980-0130.ch040 (accessed on 29 August 1980).
- Avenell, C.S.; Sainz-Diaz, C.I.; Griffiths, A.J. Solid waste pyrolysis in a pilot-scale batch pyrolyser. Fuel 1996, 75, 1167–1174. [Google Scholar] [CrossRef]
- Fortuna, F.; Cornacchia, G.; Mincarini, M.; Sharma, V.K. Pilot-scale experimental pyrolysis plant: Mechanical and operational aspects. J. Anal. Appl. Pyrolysis 1997, 40, 403–417. [Google Scholar] [CrossRef]
- Yongrong, Y.; Jizhong, C.; Guibin, Z. Technical advance on the pyrolysis of used tires in China. China-Japan International Academic SymposiumEnvironmental Problem in Chinese Iron-Steelmaking Industries and Effective Technology Transfer; Sendai, Japan, 2000; pp. 84–93. Available online: http://www2.econ.tohoku.ac.jp/~kawabata/Omurapro/omuraCDM/symp_jc/10yang.pdf (accessed on 8 April 2020).
- Li, S.Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Cen, K.F. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Díez, C.; Sánchez, M.E.; Haxaire, P.; Martínez, O.; Morán, A. Pyrolysis of tyres: A comparison of the results from a fixed-bed laboratory reactor and a pilot plant (rotatory reactor). J. Anal. Appl. Pyrolysis 2005, 74, 254–258. [Google Scholar] [CrossRef]
- Li, S.Q.; Yao, Q.; Wen, S.E.; Chi, Y.; Yan, J.H. Properties of pyrolytic chars and activated carbons derived from pilot-scale pyrolysis of used tires. J. Air Waste Manag. Assoc. 2005, 55, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Rofiqul, I.M.; Haniu, H.; Rafiqul, A.B.M. Limonene-rich liquids from pyrolysis of heavy automotive tire wastes. J. Environ. Eng. 2007, 2, 681–695. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Il, K.S.; Haniu, H.; Beg, M.R.A. Fire-tube heating pyrolysis of car tire wastes: End uses of product liquids as fuels and chemicals. Int. Energy J. 2008, 9. Available online: http://www.rericjournal.ait.ac.th/index.php/reric/article/view/485 (accessed on 8 April 2020).
- Islam, M.R.; Haniu, H.; Beg, M.R.A. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: Product yields, compositions and related properties. Fuel 2008, 87, 3112–3122. [Google Scholar]
- Islam, M.R.; Tushar, M.S.H.K.; Haniu, H. Production of liquid fuels and chemicals from pyrolysis of Bangladeshi bicycle/rickshaw tire wastes. J. Anal. Appl. Pyrolysis 2008, 82, 96–109. [Google Scholar] [CrossRef]
- Islam, M.R.; Haniu, H.; Fardoushi, J. Pyrolysis kinetics behavior of solid tire wastes available in Bangladesh. Waste Manag. 2009, 29, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Parveen, M.; Haniu, H.; Sarker, M.I. Innovation in pyrolysis technology for management of scrap tire: A solution of Energy and Environment. Int. J. Environ. Sci. Dev. 2010, 1, 89. [Google Scholar] [CrossRef]
- Donatelli, A.; Iovane, P.; Molino, A. High energy syngas production by waste tyres steam gasification in a rotary kiln pilot plant. Experimental and numerical investigations. Fuel 2010, 89, 2721–2728. [Google Scholar] [CrossRef]
- Islam, M.R.; Joardder, M.U.H.; Hasan, S.M.; Takai, K.; Haniu, H. Feasibility study for thermal treatment of solid tire wastes in Bangladesh by using pyrolysis technology. Waste Manag. 2011, 31, 2142–2149. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Sathiskumar, C.; Moorthy, R.S. Effect of process parameters on tire pyrolysis: A review. J. Sci. Ind. Res. 2012, 71, 309–315. [Google Scholar]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- Kader, M.; Islam, M.; Hossain, M.; Haniu, H. Development of a Pilot Scale Pyrolysis Plant for Production of Liquid Fuel from Waste Tire. Mech. Eng. Res. J. 2013, 9, 54–59. [Google Scholar]
- Alkhatib, R. Development of an Alternative Fuel from Waste of Used Tires by Pyrolysis. Ph.D. Thesis, L’Université Nantes Angers Le Mans France, Nantes, France, 2014. Available online: https://tel.archives-ouvertes.fr/tel-01186556/ (accessed on 5 August 2019).
- Rudniak, L.; Machniewski, P.M. Modelling and experimental investigation of waste tyre pyrolysis process in a laboratory reactor. Chem. Process Eng. 2017, 38, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Januszewicz, K.; Klein, M.; Klugmann-Radziemska, E.; Kardas, D. Thermogravimetric analysis/pyrolysis of used tyres and waste rubber. Physicochem. Probl. Miner. Process. 2017, 53, 802–811. [Google Scholar]
- Aziz, M.A.; Rahman, M.A.; Molla, H. Design, fabrication and performance test of a fixed bed batch type pyrolysis plant with scrap tire in Bangladesh. J. Radiat. Res. Appl. Sci. 2018, 11, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Diez, C.; Martinez, O.; Calvo, L.F.; Cara, J.; Morán, A. Pyrolysis of tires. Influence of the final temperature of the process on emissions and the calorific value of the products recovered. Waste Manag. 2004, 24, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Chaala, A.; Darmstadt, H. The vacuum pyrolysis of used tires: End-uses for oil and carbon black products. J. Anal. Appl. Pyrolysis 1999, 51, 201–221. [Google Scholar] [CrossRef]




| Year | Country | Description/Module | Remarks | References |
|---|---|---|---|---|
| 1980 | Japan | In 1979, the Japanese company Sumitomo Cement Co., Ltd., established a pyrolysis plant with a rotary kiln reactor with an annual capacity of 7000 tons in AΚO City, Hyogo Prefecture. | The pilot scale plant was turned into a commercial plant with low costs to recover fuel oil and carbon black. | [17,18] |
| 1996 | France | A pilot scale batch reactor was designed and used to investigate the effects of different operating conditions like temperature, fuel-to-air ratios and reaction times. | It was noticed that there were low percentages of emissions of SO2 and NOx, although the materials relatively contained high-sulfur materials. | [19] |
| 1997 | Italy | The pyrolysis process is experimentally investigated using a pilot scale reactor. | A number of gases were detected in the produced gas, such as H2, O2, N2, CO and CH4. | [20] |
| 2000 | China | This review summarizes the research about pyrolysis of waste tires in china focusing mainly on the mechanism of the pyrolysis process and the pyrolysis reactor design. | The pyrolysis industry in China has the tendency towards a small scale and low initial cost pyrolysis plant. | [21] |
| 2004 | China | The pyrolysis process is studied using a pilot scale rotary kiln reactor. The effects of operating temperatures are investigated at a range between 450 and 650 °C. | A rotary kiln is considered an excellent alternative for pyrolysis of waste tires. | [22] |
| 2005 | Spain | A comparison is made between a fixed-bed laboratory scale and a rotatory pilot scale reactor at the same operating conditions to obtain black carbon, liquid fuel, and Pyro-Gas in the two scales. | No significant differences were found between the pyrolysis black carbon obtained from tires in the two scales. | [23] |
| 2005 | China | Waste tires were subjected to a pyrolysis process using a rotary kiln pilot scale reactor. | Raw pyrolysis black carbon and activated black carbon have acceptable ability for adsorption of methylene-blue compared to commercial black carbon. | [24] |
| 2007 | Bangladesh | Heavy automotive waste tires were subjected to a pyrolysis process in a fixed-bed reactor under deferent process parameters. | Pyrolysis tire oil obtained have fuel properties that make it comparable to liquid petroleum fuels. | [25] |
| 2008 | Japan | A fixed-bed reactor that heated using a fire tube was used to pyrolyze the car tire wastes under a nitrogen condition and it was tested under different pyrolysis conditions. | The maximum liquid yield is obtained at a temperature of 475 °C. | [26] |
| 2008 | Bangladesh | The tire pyrolysis oils produced were subjected to elemental analysis, and chromatographic and spectroscopic techniques. | The tire pyrolysis oil could be used as fuel with a high heating value of 42 MJ/kg. | [27] |
| 2008 | Bangladesh | Bicycle waste tires were subjected to a pyrolysis process in a fixed-bed reactor under several process parameters. | The pyrolysis tire oil obtained could be comparable to fuels derivative from crude oil if the pyrolysis is performed under suitable operating conditions. | [28] |
| 2009 | Bangladesh | Waste tires were subjected to thermogravimetric analysis at temperatures between 30 and 800 °C and heating rates of 10 and 60 °C/min. | The pyrolysis of waste tires is significantly affected by the heating rate. | [29] |
| 2010 | Bangladesh | Pyrolysis technology using a new heating system to dispose of the solid waste tire and to recovery liquefied fuels. | The pyrolysis process is considered an environmentally friendly solution for the waste tires issue. | [30] |
| 2010 | Italy | The results of several experiments were used to develop a numerical model using the commercial code ChemCAD. | The results of an experimental and numerical study for gasification in a rotary kiln reactor of tires were explained. | [31] |
| 2011 | Bangladesh | Feasibility study was performed to obtain liquid oil, black carbon and Pyro-Gas from waste tires | The feasibility study was performed to three different scales. It can be shown that the medium commercial scale is the most economical scale. | [32] |
| 2012 | India | A review of pyrolysis of waste tires was made focusing on several operating conditions. | The pyrolysis process has three products with commercial value: black carbon, liquid fuel and Pyro-Gas. | [33] |
| 2013 | UK | This review presents that the interest in pyrolysis of waste tires to obtain products with commercial value is growing. | The Pyro-Gas produced from pyrolysis of waste tires consists of H2, CO, CO2, H2S and C1-C4 hydrocarbons. This review has focused on upgrading pyrolysis black carbon to activated black carbon and to black carbon with a better quality. | [34] |
| 2013 | Bangladesh | A small pyrolysis scale of waste tires could reduce the crisis of liquid fuel in waste in Bangladesh. | The Pyro-Gas produced from the pyrolysis of waste tires has a high heating value of 37 MJ/m3, which makes it a high enough heat source required to complete the pyrolysis process. | [5] |
| 2013 | Bangladesh | A fixed bed pilot scale reactor was used to obtain pyrolysis oil from waste tires | The properties of the pyrolysis oil were comparable to commercial diesel. | [35] |
| 2014 | France | The fuel was obtained from the pyrolysis of waste tires at optimum conditions for the temperature, heating rate and inert gas flow rate. | Without using inert gas flow, the optimum temperature for the pyrolysis process is 465 °C. | [36] |
| 2017 | Poland | The pyrolysis process for the waste tires was explained using a mathematical model. | This work shows the importance of the heat and mass. | [37] |
| 2017 | Poland | Two samples of waste tires and rubber materials were studied and compared at different heating rates (e.g., 10, 20 and 50 K/min). | The thermogravimetry equipment (TG) was used to perform a kinetic study and determine the kinetic mechanism of the process. | [38] |
| 2018 | Bangladesh | The pyro oil was produced from pyrolysis of scrap tires using a fixed-bed batch reactor. | The pyro oil produced from this reactor is used as fuel in a boiler and as furnace oil. The char is potentially used as a fertilizer, and it can be used to produce shoes and conveyor belts. | [39] |
| Products | Average (Kg) | Average (%wt) |
|---|---|---|
| Crude tire pyrolysis oil (TPO) | 2249.8 ± 63.4 | 45 ± 1.63 |
| Pyrolysis carbon black (PCB) | 1708.5 ± 102.1 | 34 ± 2.45 |
| Scrap steel | 547.5 ± 21.3 | 11 ± 0.82 |
| Pyrolysis gas (Pyro-Gas) | 494.3 ± 70.3 | 10 ± 1.83 |
| Original Tire (%wt) | Carbon Black (%wt) | |
|---|---|---|
| C 1 (%) | 84.92 | 95.42 ± 0.16 |
| H 1 (%) | 7.58 | 0.77 ± 0.20 |
| N 1 (%) | 0.51 | 0.22 ± 0.07 |
| S 1 (%) | 2.41 | 3.29 ± 0.09 |
| Cl 1 (%) | 0.05 | 0.19 ± 0.01 |
| O 1 (%) | 4.53 | 0.12 ± 0.07 |
| Ash | 7.51 | 16.5 5 ± 0.34 |
| Moisture | 0.97 | 1.16 ± 0.14 |
| Volatile matter (%) | 62.42 | 2.50 ± 0.74 |
| HHV (MJ/kg) | 35.7 | 28.70 ± 0.18 |
| Analyses | Commercial Diesel | Tire Pyrolysis Oil (TPO) |
|---|---|---|
| Elemental (wt%) | ||
| C | 86.50 | 85.69 ± 0.38 |
| H | 13.20 | 9.20 ± 0.08 |
| C/H | 6.52 | 9.32 ± 0.12 |
| N | 65 ppm < 1 | 0.59 ± 0.06 |
| S | 0.11–0.70 | 1.11 ± 0.06 |
| Ash | 0.0 | 0.13 ± 0.02 |
| O | 0.01 | 3.29 ± 0.36 |
| H/C molar ratio | 1.83 | 1.29 ± 0.02 |
| O/C molar ratio | - | 0.029 ± 0.003 |
| Empirical formula | - | CH1.29O0.029N0.006 |
| Density (kg/m3) | 820-860 | 962.5 ± 2.08 |
| Viscosity (cSt) | 2.0–4.5 a | 4.86 b ± 0.03 |
| Flash Point (°C) | >55 | 31.5 ± 1.29 |
| Pour point (°C) | - | −5.4 ± 0.48 |
| Moisture (wt%) | 79 ppm | ND |
| pH value | - | 4.32 ± 0.05 |
| HHV (MJ/kg) | 45.10 | 42.46 ± 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, R.; Juaidi, A.; Assad, M.; Salameh, T.; Manzano-Agugliaro, F. Energy Recovery from Waste Tires Using Pyrolysis: Palestine as Case of Study. Energies 2020, 13, 1817. https://0-doi-org.brum.beds.ac.uk/10.3390/en13071817
Abdallah R, Juaidi A, Assad M, Salameh T, Manzano-Agugliaro F. Energy Recovery from Waste Tires Using Pyrolysis: Palestine as Case of Study. Energies. 2020; 13(7):1817. https://0-doi-org.brum.beds.ac.uk/10.3390/en13071817
Chicago/Turabian StyleAbdallah, Ramez, Adel Juaidi, Mahmoud Assad, Tareq Salameh, and Francisco Manzano-Agugliaro. 2020. "Energy Recovery from Waste Tires Using Pyrolysis: Palestine as Case of Study" Energies 13, no. 7: 1817. https://0-doi-org.brum.beds.ac.uk/10.3390/en13071817