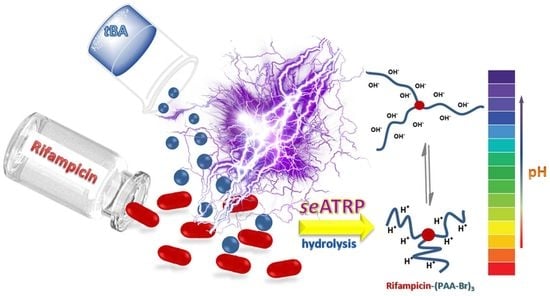
Stimuli-Responsive Rifampicin-Based Macromolecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Analysis
2.3. Synthesis of Rifampicin-Based ATRP Macroinitiator (Rif-Br3)
2.4. Electrochemical Characterization
2.5. The Synthesis of Rif-(PnBA-Br)3 by seATRP under Constant Potential Conditions with CuIIBr2/TPMA*2 Catalytic Complex
2.6. The Synthesis of Rif-(PnBA-Br)3 by seATRP under Constant Potential Conditions with CuIIBr2/TPMA Catalytic Complex
2.7. The Synthesis of Rif-(PnBA-Br)3 by Temporally-Controlled seATRP under Constant Current Conditions with CuIIBr2/TPMA Catalytic Complex
2.8. The Synthesis of Rif-(PtBA-Br)3 by seATRP under Constant Potential Conditions with CuIIBr2/TPMA Catalytic Complex
2.9. The Synthesis of Rif-(PtBA-b-PtBA-Br)3 by seATRP under Constant Potential Conditions with CuIIBr2/TPMA Catalytic Complex
2.10. Detaching Polymer Side Chains from Rifampicin-Based Macromolecules
2.11. Transformation of PtBA to PAA Side Chains
2.12. Confirmation of PtBA Hydrolysis to PAA Moieties by Contact Angle Measurements
2.13. Determination of pH-Sensitivity of PAA-Based Polymers
2.14. Loading of Quercetin (QC) into the PAA-Based Polymer and Its Release Behavior upon pH Changes
3. Results and Discussion
3.1. Synthesis and Characterization of Rifampicin-Based ATRP Macroinitiator
3.2. Synthesis of Rifmapicin-Based Macromolecules
3.3. Preparation of pH-Responsive Rifampicin-Based “Smart” Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 327–341. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Han, J.Y.; Zhao, D.D.; Li, D.; Wang, X.H.; Jin, Z.; Zhao, K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, K.; Saito, N. Biodegradable polymers as drug delivery systems for bone regeneration. Pharmaceutics 2020, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [Green Version]
- Jerca, F.A.; Jerca, V.V.; Anghelache, A.M.; Vuluga, D.M.; Hoogenboom, R. Poly(2-isopropenyl-2-oxazoline) as a versatile platform towards thermoresponsive copolymers. Polym. Chem. 2018, 9, 3473–3478. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 2016, 12, 2542–2549. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Wang, X.; Tang, J.; Yang, Y.W. Surface immobilization of pH-responsive polymer brushes on mesoporous silica nanoparticles by enzyme mimetic catalytic ATRP for controlled cargo release. Polymers 2016, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Lale, S.V.; Aswathy, R.G.; Aravind, A.; Kumar, D.S.; Koul, V. AS1411 aptamer and folic acid functionalized pH-responsive ATRP fabricated pPEGMA–PCL–pPEGMA polymeric nanoparticles for targeted drug delivery in cancer therapy. Biomacromolecules 2014, 15, 1737–1752. [Google Scholar] [CrossRef]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-responsive polymers for drug delivery: From molecular design to applications. Polym. Chem. 2014, 5, 1519–1528. [Google Scholar] [CrossRef]
- Hu, W.Y.; Wu, Z.M.; Yang, Q.Q.; Liu, Y.J.; Li, J.; Zhang, C.Y. Smart pH-responsive polymeric micelles for programmed oral delivery of insulin. Colloids Surf. B 2019, 183, 110443. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.-E.F.; Kamel, A.M.; Clarimont, C. Improving the decision-making process in the structural modification of drug candidates: Enhancing metabolic stability. Drug Discov. Today 2004, 9, 1020–1028. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Labuschagne, P.W.; Adami, R.; Liparoti, S.; Naidoo, S.; Swai, H.; Reverchon, E. Preparation of rifampicin/poly(D,L-lactice) nanoparticles for sustained release by supercritical assisted atomization technique. J. Supercrit. Fluids 2014, 95, 106–117. [Google Scholar] [CrossRef]
- Bury-Moné, S. Antibacterial therapeutic agents: Antibiotics and bacteriophages. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Chmielarz, P.; Yan, J.; Krys, P.; Wang, Y.; Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Synthesis of nanoparticle copolymer brushes via surface-initiated seATRP. Macromolecules 2017, 50, 4151–4159. [Google Scholar] [CrossRef]
- Chmielarz, P.; Pacześniak, T.; Rydel-Ciszek, K.; Zaborniak, I.; Biedka, P.; Sobkowiak, A. Synthesis of naturally-derived macromolecules through simplified electrochemically mediated ATRP. Beilstein J. Org. Chem. 2017, 13, 2466–2472. [Google Scholar] [CrossRef] [Green Version]
- Chmielarz, P. Synthesis of cationic star polymers by simplified electrochemically mediated ATRP. Express Polym. Lett. 2016, 10, 810–821. [Google Scholar] [CrossRef]
- Yin, X.; Wang, L.; Zhang, X.; Zhao, H.; Cui, Z.; Fu, P.; Liu, M.; Pang, X.; Qiao, X. Synthesis of amphiphilic star-shaped block copolymers through photo-induced metal free atom transfer radical polymerization. Eur. Polym. J. 2020, 126, 109557. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization: From mechanisms to applications. Isr. J. Chem. 2012, 52, 206–220. [Google Scholar] [CrossRef]
- Krys, P.; Matyjaszewski, K. Kinetics of atom transfer radical polymerization. Eur. Polym. J. 2017, 89, 482–523. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Fantin, M.; Pan, X.; de Fiebre, K.; Coote, M.L.; Matyjaszewski, K.; Liu, P. Mechanistically guided predictive models for ligand and initiator effects in copper-catalyzed atom transfer radical polymerization (Cu-ATRP). J. Am. Chem. Soc. 2019, 141, 7486–7497. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Advanced materials by atom transfer radical polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.d.Á.; Vieira, R.P. Current status of ATRP-based materials for gene therapy. React. Funct. Polym. 2020, 147, 104453. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of pyridoxine-based eagle-shaped asymmetric star polymers through seATRP. Polym. Adv. Technol. 2017, 28, 1787–1793. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Dually-functional riboflavin macromolecule as a supramolecular initiator and reducing agent in temporally-controlled low ppm ATRP. Express Polym. Lett. 2020, 14, 235–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, J.W.; Moretti, A.; Uhrich, K.E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control Release 2015, 219, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Zaborniak, I.; Surmacz, K.; Chmielarz, P. Synthesis of sugar-based macromolecules via sono-ATRP in miniemulsion. Polym. Adv. Technol. 2020, 31, 1972–1979. [Google Scholar] [CrossRef]
- Cuthbert, J.; Yerneni, S.S.; Sun, M.K.; Fu, T.; Matyjaszewski, K. Degradable polymer stars based on tannic acid cores by ATRP. Polymers 2019, 11, 752. [Google Scholar] [CrossRef] [Green Version]
- Zaborniak, I.; Chmielarz, P.; Flejszar, M.; Surmacz, K.; Ostatek, R. Preparation of hydrophobic tannins-inspired polymer materials via low-ppm ATRP methods. Polym. Adv. Technol. 2019, 19, 913–921. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Miniemulsion switchable electrolysis under constant current conditions. Polym. Adv. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, X.; Jiang, X.; Wang, H.; Gao, W. Modification of cellulose nanocrystal via SI-ATRP of styrene and the mechanism of its reinforcement of polymethylmethacrylate. Carbohydr. Polym. 2016, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.S.; Fein, K.; Murata, H.; Ball, R.L.; Russell, A.J.; Whitehead, K.A. ATRP-grown protein-polymer conjugates containing phenylpiperazine selectively enhance transepithelial protein transport. J. Control Release 2017, 255, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Modification of wood-based materials by atom transfer radical polymerization methods. Eur. Polym. J. 2019, 120, 109253. [Google Scholar] [CrossRef]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.D.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- Lorandi, F.; Fantin, M.; Isse, A.A.; Gennaro, A. Electrochemical triggering and control of atom transfer radical polymerization. Curr. Opin. Electrochem. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of α-D-glucose-based star polymers through simplified electrochemically mediated ATRP. Polymer 2016, 102, 192–198. [Google Scholar] [CrossRef]
- Mohapatra, H.; Kleiman, M.; Esser-Kahn, A.P. Mechanically controlled radical polymerization initiated by ultrasound. Nat. Chem. 2016, 9, 135–139. [Google Scholar] [CrossRef]
- Qu, S.W.; Wang, K.; Khan, H.; Xiong, W.F.; Zhang, W.Q. Synthesis of block copolymer nano-assemblies via ICAR ATRP and RAFT dispersion polymerization: How ATRP and RAFT lead to differences. Polym. Chem. 2019, 10, 1150–1157. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Ultrasound-mediated atom transfer radical polymerization (ATRP). Materials 2019, 12, 3600. [Google Scholar] [CrossRef] [Green Version]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; Read de Alaniz, J.; Fors, B.P.; Hawker, C.J. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkowski, A.J.; You, W.; Nicewicz, D.A. Visible light photoinitiated metal-free living cationic polymerization of 4-methoxystyrene. J. Am. Chem. Soc. 2015, 137, 7580–7583. [Google Scholar] [CrossRef] [PubMed]
- Discekici, E.H.; Anastasaki, A.; Read de Alaniz, J.; Hawker, C.J. Evolution and future directions of metal-free atom transfer radical polymerization. Macromolecules 2018, 51, 7421–7434. [Google Scholar] [CrossRef] [Green Version]
- Hong, B.M.; Park, S.A.; Park, W.H. Effect of photoinitiator on chain degradation of hyaluronic acid. Biomater. Res. 2019, 23, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, M.; Jiang, X.; Zhang, L.; Cheng, Z.; Zhu, X. Recent progress on transition metal catalyst separation and recycling in ATRP. Macromol. Rapid. Commun. 2015, 36, 1702–1721. [Google Scholar] [CrossRef]
- Kütahya, C.; Schmitz, C.; Strehmel, V.; Yagci, Y.; Strehmel, B. Near-infrared sensitized photoinduced atom-transfer radical polymerization (ATRP) with a copper(II) catalyst concentration in the ppm range. Angew. Chem. Int. Ed. 2018, 57, 7898–7902. [Google Scholar] [CrossRef]
- Trevisanello, E.; De Bon, F.; Daniel, G.; Lorandi, F.; Durante, C.; Isse, A.A.; Gennaro, A. Electrochemically mediated atom transfer radical polymerization of acrylonitrile and poly(acrylonitrile-b-butyl acrylate) copolymer as a precursor for N-doped mesoporous carbons. Electrochim. Acta 2018, 285, 344–354. [Google Scholar] [CrossRef]
- Wang, J.; Tian, M.; Li, S.; Wang, R.; Du, F.; Xue, Z. Ligand-free iron-based electrochemically mediated atom transfer radical polymerization of methyl methacrylate. Polym. Chem. 2018, 9, 4386–4394. [Google Scholar] [CrossRef]
- Michieletto, A.; Lorandi, F.; De Bon, F.; Isse, A.A.; Gennaro, A. Biocompatible polymers via aqueous electrochemically mediated atom transfer radical polymerization. J. Polym. Sci. 2020, 58, 114–123. [Google Scholar] [CrossRef]
- De Bon, F.; Isse, A.A.; Gennaro, A. Electrochemically mediated atom transfer radical polymerization of methyl methacrylate: The importance of catalytic halogen exchange. ChemElectroChem 2019, 6, 4257–4265. [Google Scholar] [CrossRef]
- De Bon, F.; Isse, A.A.; Gennaro, A. Towards scale-up of electrochemically-mediated atom transfer radical polymerization: Use of a stainless-steel reactor as both cathode and reaction vessel. Electrochim. Acta 2019, 304, 505–512. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, X.W.; Hu, J.; Bo, S.; Zhang, J.M.; Wang, W.; Li, S.Y.; Yang, Y.F. Preparation of hemoglobin (Hb)-imprinted poly(ionic liquid)s via Hb-catalyzed eATRP on gold nanodendrites. Anal. Bioanal. Chem. 2020, 412, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chmielarz, P.; Gennaro, A.; Matyjaszewski, K. Simplified electrochemically mediated atom transfer radical polymerization using a sacrificial anode. Angew. Chem. Int. Ed. 2015, 54, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Fantin, M.; Lorandi, F.; Isse, A.A.; Gennaro, A. Sustainable electrochemically-mediated atom transfer radical polymerization with inexpensive non-platinum electrodes. Macromol. Rapid. Commun. 2016, 37, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- De Bon, F.; Fantin, M.; Isse, A.A.; Gennaro, A. Electrochemically mediated ATRP in ionic liquids: Controlled polymerization of methyl acrylate in [BMIm][OTf]. Polym. Chem. 2018, 9, 646–655. [Google Scholar] [CrossRef]
- Xia, J.H.; Matyjaszewski, K. Controlled/“living” radical polymerization. Atom transfer radical polymerization catalyzed by copper(I) and picolylamine complexes. Macromolecules 1999, 32, 2434–2437. [Google Scholar] [CrossRef]
- Kaur, A.; Ribelli, T.G.; Schröder, K.; Matyjaszewski, K.; Pintauer, T. Properties and ATRP activity of copper complexes with substituted tris(2-pyridylmethyl)amine-based ligands. Inorg. Chem. 2015, 54, 1474–1486. [Google Scholar] [CrossRef]
- Wang, Y.; Lorandi, F.; Fantin, M.; Chmielarz, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Miniemulsion ARGET ATRP via interfacial and ion-pair catalysis: From ppm to ppb of residual copper. Macromolecules 2017, 50, 8417–8425. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Wolski, K.; Grześ, G.; Isse, A.A.; Gennaro, A.; Zapotoczny, S.; Sobkowiak, A. Tannic acid-inspired star-like macromolecules via temporally controlled multi-step potential electrolysis. Macromol. Chem. Phys. 2019, 220, 1900073. [Google Scholar] [CrossRef]
- Wang, D.; Tan, J.; Kang, H.; Ma, L.; Jin, X.; Liu, R.; Huang, Y. Synthesis, self-assembly and drug release behaviors of pH-responsive copolymers ethyl cellulose-graft-PDEAEMA through ATRP. Carbohydr. Polym. 2011, 84, 195–202. [Google Scholar] [CrossRef]
- Taguchi, M.; Yamane, Y.; Aikawa, N.; Tsukamoto, G. SV 1H-and 13C-Nuclear Magnetic Resonance Spectra of Rifampicin and 3-[(Dimethylhydrazono) methyl] rifamycin SV. Chem. Pharm. Bull. 1988, 36, 4157–4161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielarz, P. Cellulose-based graft copolymers prepared by simplified electrochemically mediated ATRP. Express Polym. Lett. 2017, 11, 140–151. [Google Scholar] [CrossRef]
- Horn, M.; Matyjaszewski, K. Solvent Effects on the Activation rate constant in atom transfer radical polymerization. Macromolecules 2013, 46, 3350–3357. [Google Scholar] [CrossRef]
- Wang, J.; Han, J.; He, D.; Peng, H.; Xue, Z.; Xie, X. Active, effective, and “green” iron(III)/polar solvent catalysts for AGET ATRP of methyl methacrylate with various morphologies of elemental silver as a reducing agent. RSC Adv. 2016, 6, 88490–88497. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.-M. Multielectron, multistep molecular catalysis of electrochemical reactions: Benchmarking of homogeneous catalysts. ChemElectroChem 2014, 1, 1226–1236. [Google Scholar] [CrossRef]
- Magenau, A.J.D.; Bortolamei, N.; Frick, E.; Park, S.; Gennaro, A.; Matyjaszewski, K. Investigation of electrochemically mediated atom transfer radical polymerization. Macromolecules 2013, 46, 4346–4353. [Google Scholar] [CrossRef]
- Tang, W.; Matyjaszewski, K. Kinetic modeling of normal ATRP, normal ATRP with [CuII]0, reverse ATRP and SR&NI ATRP. Macromol. Theory Simul. 2008, 17, 359–375. [Google Scholar] [CrossRef]
- Mastan, E.; Zhu, S. A molecular weight distribution polydispersity equation for the ATRP system: Quantifying the effect of radical termination. Macromolecules 2015, 48, 6440–6449. [Google Scholar] [CrossRef]
- Vidal, J.; Villegas, L.; Peralta-Hernández, J.M.; Salazar González, R. Removal of Acid Black 194 dye from water by electrocoagulation with aluminum anode. J. Environ. Sci. Health A 2016, 51, 289–296. [Google Scholar] [CrossRef]
- Shanti, R.; Bella, F.; Salim, Y.S.; Chee, S.Y.; Ramesh, S.; Ramesh, K. Poly(methyl methacrylate-co-butyl acrylate-co-acrylic acid): Physico-chemical characterization and targeted dye sensitized solar cell application. Mater. Des. 2016, 108, 560–569. [Google Scholar] [CrossRef]
- Xie, Y.; Moreno, N.; Calo, V.M.; Cheng, H.; Hong, P.-Y.; Sougrat, R.; Behzad, A.R.; Tayouo, R.; Nunes, S.P. Synthesis of highly porous poly(tert-butyl acrylate)-b-polysulfone-b-poly(tert-butyl acrylate) asymmetric membranes. Polym. Chem. 2016, 7, 3076–3089. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Ghosh, P.; Dhara, S.; Panda, A.B.; Pal, S. Dextrin and poly(acrylic acid)-based biodegradable, non-cytotoxic, chemically cross-linked hydrogel for sustained release of ornidazole and ciprofloxacin. ACS Appl. Mater. Interfaces 2015, 7, 4791–4803. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, C.; Hasirci, N. Evaluation of surface free energy for PMMA films. J. Appl. Polym. Sci. 2008, 108, 438–446. [Google Scholar] [CrossRef]
- Król, P.; Chmielarz, P. Synthesis of polystyrene-b-polyurethane-b-polystyrene copolymers through ARGET ATRP polymerization method. Part II. Chemical structure, thermal and surface properties. Polimery 2015, 60, 377–384. [Google Scholar] [CrossRef]






| Entry | [Monomer]0/[Initiator]0/[CuL] | Ligand | Monomer | Initiator | [CuL] | Conv b (%) | kpapp b (h−1) | DPn,theo b (per arm) | Mn,theoc (×10−3) | Mn,appd (×10−3) | Mw/Mnd | dvolumee (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 270/1/0.11 | TPMA*2 | nBA | Rif-Br3 | 400 | 48 | 0.133 | 130 | 51.1 | 26.1 | 1.63 | 12 ± 1 |
| 2 | 270/1/0.11 | TPMA | nBA | Rif-Br3 | 400 | 51 | 0.109 | 137 | 54.1 | 36.4 | 1.29 | 16 ± 1 |
| 3 f | 270/1/0.11 | TPMA | nBA | Rif-Br3 | 400 | 53 | 0.095 g | 144 | 56.1 | 26.2 | 1.59 | 15 ± 2 |
| 4 | 101/1/0.04 | TPMA | tBA | Rif-Br3 | 400 | 76 | 0.137 | 77 | 30.1 | 17.6 | 1.71 | 9 ± 1 |
| 5 | 182/1/0.07 | TPMA | tBA | Rif-(PtBA-Br)3 from entry 4 | 400 | 59 | 0.097 | 107 | 72.1 | 25.6 | 1.58 | 14 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaborniak, I.; Macior, A.; Chmielarz, P. Stimuli-Responsive Rifampicin-Based Macromolecules. Materials 2020, 13, 3843. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13173843
Zaborniak I, Macior A, Chmielarz P. Stimuli-Responsive Rifampicin-Based Macromolecules. Materials. 2020; 13(17):3843. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13173843
Chicago/Turabian StyleZaborniak, Izabela, Angelika Macior, and Paweł Chmielarz. 2020. "Stimuli-Responsive Rifampicin-Based Macromolecules" Materials 13, no. 17: 3843. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13173843