Confined Polysulfides in N-Doped 3D-CNTs Network for High Performance Lithium-Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3D-NCNT-Li2S6 Composite
2.2. Materials Characterizations
2.3. Electrochemical Measurements
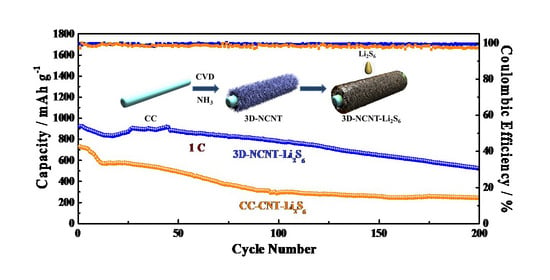
3. Results and Discussion
3.1. Structural Characterization
3.2. Evaluation of Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.Z.; Su, Y.S. Challenges and prospects of lithium–sulfur batteries. Acc. Chem. Res. 2012, 46, 1125–1134. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.H.; Deng, S.J.; Zhan, J.Y.; Fang, R.Y.; Xia, Y.; Wang, X.L.; Zhang, Q.; Tu, J.P. Popcorn inspired porous macrocellular carbon: Rapid puffing fabrication from rice and its applications in lithium–sulfur batteries. Adv. Energy Mater. 2017, 8, 1701110. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Tu, J.P.; Lu, Y.; Cai, J.B.; Zhang, Y.J.; Wang, X.L.; Gu, C.D. Graphene-coated mesoporous carbon/sulfur cathode with enhanced cycling stability. Electrochim. Acta 2013, 113, 256–262. [Google Scholar] [CrossRef]
- Nitti, A.; Forti, G.; Bianchi, G.; Botta, C.; Tinti, F.; Gazzano, M.; Camaioni, N.; Po, R.; Pasini, D. Anthradithiophene-based organic semiconductors through regiodirected double annulations. J. Mater. Chem. C 2021, 9, 9302–9308. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Drosos, C.; Jia, C.; Mathew, S.; Palgrave, R.G.; Moss, B.; Kafizas, A.; Vernardou, D. Aerosol-assisted chemical vapor deposition of V2O5 cathodes with high rate capabilities for magnesium-ion batteries. J. Power Sources 2018, 384, 355–359. [Google Scholar] [CrossRef]
- Callegari, D.; Colombi, S.; Nitti, A.; Simari, C.; Nicotera, I.; Ferrara, C.; Mustarelli, P.; Pasini, D.; Quartarone, E. Autonomous Self-Healing Strategy for Stable Sodium-Ion Battery: A Case Study of Black Phosphorus Anodes. ACS Appl. Mater. Interfaces 2021, 13, 13170–13182. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.Q.; Wang, X.L.; Wang, D.H.; Li, Y.; Zhang, Y.J.; Yang, T.; Yu, T.; Tu, J.P. Metal hydroxide–a new stabilizer for the construction of sulfur/carbon composites as high-performance cathode materials for lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 17106–17112. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.Y.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Li, S.H.; Wang, X.H.; Xia, X.H.; Wang, Y.D.; Wang, X.L.; Tu, J.P. Sulfur cathode integrated with multileveled carbon nanoflake-nanosphere networks for high-performance lithium-sulfur batteries. Electrochim. Acta 2017, 227, 217–224. [Google Scholar] [CrossRef]
- Xu, G.Y.; Ding, B.; Pan, J.; Nie, P.; Shen, L.F.; Zhang, X.G. High performance lithium-sulfur batteries: Advances and challenges. J. Mater. Chem. A 2014, 2, 12662–12676. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.M.; Yuan, L.X.; Hao, Z.X.; Huang, Y.H. Status and prospects in sulfur–carbon composites as cathode materials for rechargeable lithium–sulfur batteries. Carbon 2015, 92, 41–63. [Google Scholar] [CrossRef]
- Shan, X.; Zhong, Y.; Zhang, L.; Zhang, Y.; Xia, X.; Wang, X.; Tu, J. A Brief Review on Solid Electrolyte Interphase Composition Characterization Technology for Lithium Metal Batteries: Challenges and Perspectives. J. Phys. Chem. C 2021, 125, 19060–19080. [Google Scholar] [CrossRef]
- Niu, X.Q.; Wang, X.L.; Xie, D.; Wang, D.H.; Zhang, Y.D.; Li, Y.; Yu, T.; Tu, J.P. Nickel hydroxide-modified sulfur/carbon composite as a high-performance cathode material for lithium sulfur battery. ACS Appl. Mater. Interfaces 2015, 7, 16715–16722. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Nazar, L.F. Long-life and high-areal-capacity Li-S batteries enabled by a light-weight polar host with intrinsic polysulfide adsorption. ACS Nano 2016, 10, 4111–4118. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Z.W.; Peng, H.J.; Huang, J.Q.; Zhang, Q. A toolbox for lithium–sulfur battery research: Methods and protocols. Small Methods 2017, 1, 1700134. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.B.; Yuan, C.; Guo, Z.; Lou, X.W. Confining sulfur in double-shelled hollow carbon spheres for lithium–sulfur batteries. Angew. Chem. 2012, 124, 9730–9733. [Google Scholar] [CrossRef]
- Cao, R.G.; Xu, W.; Lv, D.P.; Xiao, J.; Zhang, J.G. Anodes for rechargeable lithium-sulfur batteries. Adv. Energy Mater. 2015, 5, 1402273. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Pope, M.A.; Aksay, I.A. Structural design of cathodes for Li-S batteries. Adv. Energy Mater. 2015, 5, 1500124. [Google Scholar] [CrossRef]
- Zhang, S.S. A new finding on the role of LiNO3 in lithium-sulfur battery. J. Power Sources 2016, 322, 99–105. [Google Scholar] [CrossRef]
- Zhang, S.S. Role of LiNO3 in rechargeable lithium/sulfur battery. Electrochim. Acta 2012, 70, 344–348. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; He, Y.; Liu, N.; Tan, T.; Liang, C. Biomass derived nitrogen-doped highly porous carbon material with a hierarchical porous structure for high-performance lithium/sulfur batteries. Materials 2017, 10, 1158. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, T.; Jeong, Y.C.; Lee, K.; Park, K.T.; Yang, S.J.; Park, C.R. Stabilization of insoluble discharge products by facile aniline modification for high performance Li-S batteries. Adv. Energy Mater. 2015, 5, 150028. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, W.; Jang, J.; Manthiram, A. Sulfur-embedded activated multichannel carbon nanofiber composites for long-life, high-rate lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1601943. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhang, Z.; Qu, Y.H.; Lai, Y.Q.; Li, J. Nitrogen-doped graphene/sulfur composite as cathode material for high capacity lithium-sulfur batteries. J. Power Sources 2014, 256, 361–368. [Google Scholar] [CrossRef]
- Wu, M.; Cui, Y.; Fu, Y. Li2S nanocrystals confined in free-standing carbon paper for high performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2015, 7, 21479–21486. [Google Scholar] [CrossRef]
- Cen, T.; Zhang, Y.; Tian, Y.; Zhang, X. Synthesis and electrochemical performance of graphene@halloysite nanotubes/sulfur composites cathode materials for lithium-sulfur batteries. Materials 2020, 13, 5158. [Google Scholar] [CrossRef]
- Jin, F.; Xiao, S.; Lu, L.; Wang, Y. Efficient activation of high-loading sulfur by small CNTs confinedinside a large CNT for high-capacity and high-rate lithium-sulfur batteries. Nano Lett. 2016, 16, 440–447. [Google Scholar] [CrossRef]
- Hu, G.; Sun, Z.; Shi, C.; Fang, R.; Chen, J.; Hou, P.; Liu, C.; Cheng, H.M.; Li, F. A sulfur-rich copolymer@CNT hybrid cathode with dual-confinement of polysulfides for high-performance lithium-sulfur batteries. Adv. Mater. 2017, 29, 1603835. [Google Scholar] [CrossRef]
- Hong, X.H.; Jin, J.; Wu, T.; Lu, Y.; Zhang, S.P.; Chen, C.H.; Wen, Z.Y. rGO-CNT aero gel covalently bonded with a nitrogen-rich polymer as a polysulfide adsorptive cathode for high sulfur loading lithium sulfur battery. J. Mater. Chem. A 2017, 5, 14775–14782. [Google Scholar] [CrossRef]
- Niu, S.; Lv, W.; Zhou, G.; He, Y.; Li, B.; Yang, Q.H.; Kang, F. N and S co-doped porous carbon spheres prepared using L-cysteine as a dual functional agent for high-performance lithium-sulfur batteries. Chem. Commun. 2015, 51, 17720–17723. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.H.; Liu, Y.; Liu, M.; Wong, C.P. 3D Nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energy 2013, 2, 241–248. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Wickramaratne, N.P.; Jaroniec, M.; Dou, S.; Dai, L. High-performance sodium ion batteries based on a 3D anode from nitrogen-doped graphene foams. Adv. Mater. 2015, 27, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, L.; Ren, J.; Zhang, M.; Luo, X.; Li, B.; Song, Z.; Zhou, X. Wheat straw-derived N-, O-, and S-Tri-doped porous carbon with ultrahigh specific surface area for lithium-sulfur batteries. Materials 2018, 11, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Huang, H.; Bae, J.; Chung, S.H.; Zhang, W.K.; Manthiram, A.; Yu, G.H. Nanostructured host materials for trapping sulfur in rechargeable Li-S batteries: Structure design and interfacial chemistry. Small Methods 2018, 2, 1700279. [Google Scholar] [CrossRef]
- Li, C.; Xi, Z.; Guo, D.; Chen, X.; Yin, L. Chemical immobilization effect on lithium polysulfides for lithium-sulfur batteries. Small 2018, 14, 1701986. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.X.; Magasinski, A.; Yushin, G. Nanoporous Li2S and MWCNT-linked Li2S powder cathodes for lithium-sulfur and lithium-ion battery chemistries. J. Mater. Chem. A 2014, 2, 6064–6070. [Google Scholar] [CrossRef]
- He, J.R.; Chen, Y.F.; Lv, W.G.; Wen, K.C.; Xu, C.; Zhang, W.L.; Qin, W.; He, W.D. Three-dimensional CNT/graphene-Li2S aerogel as freestanding cathode for high-performance Li-S batteries. ACS Energy Lett. 2016, 1, 820–826. [Google Scholar] [CrossRef]
- Ye, X.M.; Ma, J.; Hu, Y.S.; Wei, H.Y.; Ye, F.F. MWCNTs porous microspheres with efficient 3D conductive network for high performance lithium-sulfur batteries. J. Mater. Chem. A 2015, 4, 775–780. [Google Scholar] [CrossRef]
- Wu, H.L.; Xia, L.; Ren, J.; Zheng, Q.J.; Xu, C.G.; Lin, D.M. A high-efficiency N/P co-doped graphene/CNT@porous carbon hybrid matrix as a cathode host for high performance lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 20458–20472. [Google Scholar] [CrossRef]
- Wang, X.; Gao, T.; Han, F.; Ma, Z.; Zhang, Z.; Li, J.; Wang, C. Stabilizing high sulfur loading Li–S batteries by chemisorption of polysulfide on three-dimensional current collector. Nano Energy 2016, 30, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Zhang, J.; Zhang, Y.J.; Xiong, Q.Q.; Tong, Y.Y.; Li, Y.; Wang, X.L.; Gu, C.D.; Tu, J.P. Porous reduced graphene oxide sheet wrapped silicon composite fabricated by steam etching for lithium-ion battery application. J. Power Sources 2015, 286, 431–437. [Google Scholar] [CrossRef]
- Su, D.W.; Cortie, M.; Wang, G.X. Fabrication of N-doped graphene-carbon nanotube hybrids from prussian blue for lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1602014. [Google Scholar] [CrossRef]
- Ji, J.; Liu, J.; Lai, L.; Zhao, X.; Zhen, Y.; Lin, J.; Zhu, Y.; Ji, H.; Zhang, L.L.; Ruoff, R.S. In situ activation of nitrogen-doped graphene anchored on graphite foam for a high-capacity anode. ACS Nano 2015, 9, 8609–8616. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kirk, C.; Cai, Q.; Cuadrado Collados, C.; Silvestre Albero, J.; Rodríguez Reinoso, F.; Biggs, M.J. A high-volumetric-capacity cathode based on interconnected close-packed N-doped porous carbon nanospheres for long-life lithium–sulfur batteries. Adv. Energy Mater. 2017, 7, 1701082. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Ding, Y.; Peng, L.L.; Zhang, W.K.; Yu, G.H. A conductive molecular framework derived Li2S/N,P-codoped carbon cathode for advanced lithium–sulfur batteries. Adv. Energy Mater. 2017, 7, 1602876. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, L.; Mukherjee, S.; Gao, J.; Sun, H.; Liu, Z.; Ma, X.; Gupta, T.; Singh, C.V.; Ren, W.; et al. Phosphorene as a polysulfide immobilizer and catalyst in high-performance lithium–sulfur batteries. Adv. Mater. 2016, 29, 1602734. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.E.; Guan, J.D.; Feng, Q.J.; Wu, X.W.; Xiao, Z.B.; Zhang, W.; Tong, S.; Zhou, N.; Gong, D.X. Accelerated polysulfide redox kinetics revealed by ternary sandwich-type S@Co/N-doped carbon nanosheet for high-performance lithium-sulfur batteries. Carbon 2018, 128, 86–96. [Google Scholar] [CrossRef]
- Wang, D.H.; Xie, D.; Xia, X.H.; Zhang, X.Q.; Tang, W.J.; Zhong, Y.; Wu, J.B.; Wang, X.L.; Tu, J.P. A 3D conductive network with high loading Li2S@C for high performance lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 19358–19363. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Yin, L.; Koratkar, N.; Li, F.; Cheng, H.-M. Stabilizing sulfur cathodes using nitrogen-doped graphene as a chemical immobilizer for Li S batteries. Carbon 2016, 108, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Zhou, X.; Zhang, L.; Xin, X.; Wang, G.; Liu, Z. Sulfur/Carbon Nanotube Composite Film as a Flexible Cathode for Lithium–Sulfur Batteries. J. Phys. Chem. C 2013, 117, 21112–21119. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, Z.; Nie, H.; Lu, Y.; Yang, K.; Huang, S. Porous carbon nanotubes etched by water steam for high-rate large-capacity lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 8683–8689. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Huang, J.-Q.; Zhang, Q.; Peng, H.-J.; Zhao, M.-Q.; Wei, F. Aligned carbon nanotube/sulfur composite cathodes with high sulfur content for lithium–sulfur batteries. Nano Energy 2014, 4, 65–72. [Google Scholar] [CrossRef]
- Ahn, W.; Kim, K.-B.; Jung, K.-N.; Shin, K.-H.; Jin, C.-S. Synthesis and electrochemical properties of a sulfur-multi walled carbon nanotubes composite as a cathode material for lithium sulfur batteries. J. Power Sources 2012, 202, 394–399. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, W.; Cheng, X.-B.; Huang, J.-Q.; Peng, H.-J.; Yang, S.-H.; Zhang, Q. Cathode materials based on carbon nanotubes for high-energy-density lithium–sulfur batteries. Carbon 2014, 75, 161–168. [Google Scholar] [CrossRef]
- Benítez, A.; Caballero, A.; Morales, J.; Hassoun, J.; Rodríguez-Castellón, E.; Canales-Vázquez, J. Physical activation of graphene: An effective, simple and clean procedure for obtaining microporous graphene for high-performance Li/S batteries. Nano Res. 2019, 12, 759–766. [Google Scholar] [CrossRef]
- Zegeye, T.A.; Tsai, M.-C.; Cheng, J.-H.; Lin, M.-H.; Chen, H.-M.; Rick, J.; Su, W.-N.; Kuo, C.-F.J.; Hwang, B.-J. Controllable embedding of sulfur in high surface area nitrogen doped three dimensional reduced graphene oxide by solution drop impregnation method for high performance lithium-sulfur batteries. J. Power Sources 2017, 353, 298–311. [Google Scholar] [CrossRef]
- Benítez, A.; Di Lecce, D.; Caballero, Á.; Morales, J.; Rodríguez-Castellón, E.; Hassoun, J. Lithium sulfur battery exploiting material design and electrolyte chemistry: 3D graphene framework and diglyme solution. J. Power Sources 2018, 397, 102–112. [Google Scholar] [CrossRef]






| Cathode | Rate | Initial Discharge Capacity (mAh g−1) | Stable Discharge Capacity (mAh g−1) and Cycles |
|---|---|---|---|
| S-CNT [55] | 0.1C | 1109 | 740 after 100 |
| PCNT-S [56] | 0.1C | 895 | 625 after 100 |
| CNT-S [57] | 0.1C | 736.8 | 408.4 after 85 |
| S-MWCTs [58] | 100 mA/g | 1330 | 854 after 30 |
| CNT/S [59] | 0.1C | 864 | 358 after 100 |
| A-3DNG/S [60] | 0.2C | 1101 | 860 after 200 |
| N-G-S [54] | 0.3A/g | 1150 | 880 after 100 |
| S@N-3D-rGO [61] | 0.2C | 1042 | 987 after 100 |
| 3DNG-S [62] | 0.2C | 1050 | 990 after 100 |
| 3D-NCNT-Li2S6 (This work) | 0.1C | 1170.8 | 769.7 after 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhou, A.; Yao, Z.; Xia, X.; Zhang, Y. Confined Polysulfides in N-Doped 3D-CNTs Network for High Performance Lithium-Sulfur Batteries. Materials 2021, 14, 6131. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14206131
Wang D, Zhou A, Yao Z, Xia X, Zhang Y. Confined Polysulfides in N-Doped 3D-CNTs Network for High Performance Lithium-Sulfur Batteries. Materials. 2021; 14(20):6131. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14206131
Chicago/Turabian StyleWang, Donghuang, Aijun Zhou, Zhujun Yao, Xinhui Xia, and Yongqi Zhang. 2021. "Confined Polysulfides in N-Doped 3D-CNTs Network for High Performance Lithium-Sulfur Batteries" Materials 14, no. 20: 6131. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14206131