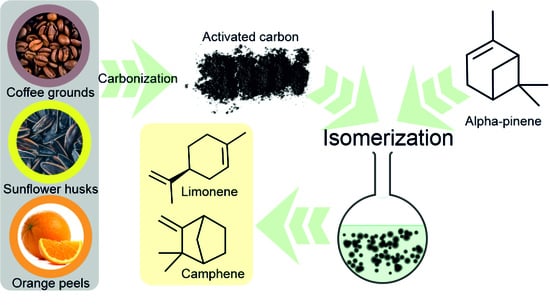
Activated Carbons Obtained from Orange Peels, Coffee Grounds, and Sunflower Husks—Comparison of Physicochemical Properties and Activity in the Alpha-Pinene Isomerization Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Raw Materials of Natural Origin for the Process of Chemical Activation and Carbonization
2.2. Chemical Activation and Carbonization of Biomass
2.3. Characterization the Obtained Activated Carbons
2.4. Alpha-Pinene Isomerization Method
3. Results and Discussion
3.1. Characterization of the Obtained Materials
3.2. Activity of Activated Carbons
3.3. Determination of the Kinetics Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jędrzak, A.; Rębiś, T.; Klapiszewski, Ł.; Zdarta, J.; Milczarek, G.; Jesionowski, T. Carbon paste electrode based on functional GOx/silica-lignin system to prepare an amperometric glucose biosensor. Sensors Actuators B Chem. 2018, 256, 176–185. [Google Scholar] [CrossRef]
- Eriksen, M.H.; Damgaard, C.K.; Christensen, L.H.; McKinnon, D.; Kleveland, K.; Ouacha, M.; Doverfelt, S.; Merta, E.; Arnold, M. Barriers for utilisation of biowaste; TemaNord; Nordic Council of Ministers: Copenhagen, Denmark, 2017; ISBN 9789289349062. [Google Scholar]
- Martin, M.J.; Artola, A.; Balaguer, M.D.; Rigola, M. Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. Chem. Eng. J. 2003, 94, 231–239. [Google Scholar] [CrossRef]
- Żółtowska, S.; Bielan, Z.; Zembrzuska, J.; Siwińska-Ciesielczyk, K.; Piasecki, A.; Zielińska-Jurek, A.; Jesionowski, T. Modification of structured bio-carbon derived from spongin-based scaffolds with nickel compounds to produce a functional catalyst for reduction and oxidation reactions: Potential for use in environmental protection. Sci. Total Environ. 2021, 794, 148692. [Google Scholar] [CrossRef]
- Kishibayev, K.K.; Serafin, J.; Tokpayev, R.R.; Khavaza, T.N.; Atchabarova, A.A.; Abduakhytova, D.A.; Ibraimov, Z.T.; Sreńscek-Nazzal, J. Physical and chemical properties of activated carbon synthesized from plant wastes and shungite for CO2 capture. J. Environ. Chem. Eng. 2021, 9, 106798. [Google Scholar] [CrossRef]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Ouzzine, M.; Serafin, J.; Sreńscek-Nazzal, J. Single step preparation of activated biocarbons derived from pomegranate peels and their CO2 adsorption performance. J. Anal. Appl. Pyrolysis 2021, 160, 105338. [Google Scholar] [CrossRef]
- Daouda, M.M.A.; Akowanou, A.V.O.; Mahunon, S.E.R.; Adjinda, C.K.; Aina, M.P.; Drogui, P. Optimal removal of diclofenac and amoxicillin by activated carbon prepared from coconut shell through response surface methodology. S. Afr. J. Chem. Eng. 2021, 38, 78–89. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kadirvelu, K. Activated carbons prepared from coir pith by physical and chemical activation methods. Bioresour. Technol. 1997, 62, 123–127. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Cruz, O.F.; Sreńscek-Nazzal, J.; Campello Gómez, I.; Azar, F.-Z.; Rey Mafull, C.A.; Hotza, D.; Rambo, C.R. Conversion of fruit waste-derived biomass to highly microporous activated carbon for enhanced CO2 capture. Waste Manag. 2021, 136, 273–282. [Google Scholar] [CrossRef]
- Daud, W.M.A.W.; Ali, W.S.W.; Sulaiman, M.Z. The effects of carbonization temperature on pore development in palm-shell-based activated carbon. Carbon N. Y. 2000, 38, 1925–1932. [Google Scholar] [CrossRef]
- Herawan, S.G.; Hadi, M.S.; Ayob, M.R.; Putra, A. Characterization of Activated Carbons from Oil-Palm Shell by CO 2 Activation with No Holding Carbonization Temperature. Sci. World J. 2013, 2013, 624865. [Google Scholar] [CrossRef] [Green Version]
- Puig-Gamero, M.; Esteban-Arranz, A.; Sanchez-Silva, L.; Sánchez, P. Obtaining activated biochar from olive stone using a bench scale high-pressure thermobalance. J. Environ. Chem. Eng. 2021, 9, 105374. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Van Thuan, T.; Quynh, B.T.P.; Nguyen, T.D.; Ho, V.T.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Depci, T.; Kul, A.R.; Önal, Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: Study in single- and multi-solute systems. Chem. Eng. J. 2012, 200–202, 224–236. [Google Scholar] [CrossRef]
- Tian, X.; Ma, H.; Li, Z.; Yan, S.; Ma, L.; Yu, F.; Wang, G.; Guo, X.; Ma, Y.; Wong, C. Flute type micropores activated carbon from cotton stalk for high performance supercapacitors. J. Power Sources 2017, 359, 88–96. [Google Scholar] [CrossRef]
- Saeidian, H.; Khajeh, S.V.; Mirjafary, Z.; Eftekhari-Sis, B. Immobilized copper nanoparticles on nitrogen-rich porous activated carbon from egg white biomass: A robust hydrophilic–hydrophobic balance catalyst for click reaction. RSC Adv. 2018, 8, 38801–38807. [Google Scholar] [CrossRef] [Green Version]
- Szatkowski, T.; Kopczyński, K.; Motylenko, M.; Borrmann, H.; Mania, B.; Graś, M.; Lota, G.; Bazhenov, V.V.; Rafaja, D.; Roth, F.; et al. Extreme biomimetics: A carbonized 3D spongin scaffold as a novel support for nanostructured manganese oxide(IV) and its electrochemical applications. Nano Res. 2018, 11, 4199–4214. [Google Scholar] [CrossRef]
- Teixeira, F.; dos Santos, B.A.; Nunes, G.; Soares, J.M.; do Amaral, L.A.; de Souza, G.H.O.; de Resende, J.T.V.; Menegassi, B.; Rafacho, B.P.M.; Schwarz, K.; et al. Addition of Orange Peel in Orange Jam: Evaluation of Sensory, Physicochemical, and Nutritional Characteristics. Molecules 2020, 25, 1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, M.A.; Siles, J.A.; Chica, A.F.; Martín, A. Biomethanization of orange peel waste. Bioresour. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef]
- Santos, C.M.; Dweck, J.; Viotto, R.S.; Rosa, A.H.; de Morais, L.C. Application of orange peel waste in the production of solid biofuels and biosorbents. Bioresour. Technol. 2015, 196, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged Citric Acid Fermentation on Orange Peel Autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef]
- Kosseva, M.R. Sources, characteristics and treatment of plant-based food waste. In Food Industry Wastes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–66. [Google Scholar]
- De Medina-Salas, L.; Giraldi-Díaz, M.R.; Castillo-González, E.; Morales-Mendoza, L.E. Valorization of orange peel waste using precomposting and vermicomposting processes. Sustainability 2020, 12, 7626. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crops Prod. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Arie, A.A.; Kristianto, H.; Suharto, I.; Halim, M.; Lee, J.K. Preparation of Orange Peel Based Activated Carbons as Cathodes in Lithium Ion Capacitors. Adv. Mater. Res. 2014, 896, 95–99. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, Z.; Cheng, Y.; Wang, X.; Yang, X.; Wang, Z. Preparation and electrochemical performance of orange peel based-activated carbons activated by different activators. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 221–227. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Pirajan, J.C. Activated Carbon Prepared From Orange Peels Coated With Titanium Oxide Nanoparticles: Characterization and Applications in the Decomposition of NOx. Orient. J. Chem. 2014, 30, 451–461. [Google Scholar] [CrossRef]
- Pan, H.; Sun, J.; Liu, J.; Zhang, Y.; Zhou, S. Preparation of sulfonated carbon derived from orange peel and its application in esterification. Chem. Phys. Lett. 2021, 770, 138395. [Google Scholar] [CrossRef]
- Arcibar-Orozco, J.A.; Rangel-Mendez, J.R.; Bandosz, T.J. Reactive adsorption of SO2 on activated carbons with deposited iron nanoparticles. J. Hazard. Mater. 2013, 246–247, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, P.; Veeramani, V.; Chen, S.M.; Madhu, R.; Liu, S. Bin Palladium Nanoparticle Incorporated Porous Activated Carbon: Electrochemical Detection of Toxic Metal Ions. ACS Appl. Mater. Interfaces 2016, 8, 1319–1326. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef]
- Roussos, S.; de los Angeles Aquiáhuatl, M.; del Refugio Trejo-Hernández, M.; Gaime Perraud, I.; Favela, E.; Ramakrishna, M.; Raimbault, M.; Viniegra-González, G. Biotechnological management of coffee pulp - isolation, screening, characterization, selection of caffeine-degrading fungi and natural microflora present in coffee pulp and husk. Appl. Microbiol. Biotechnol. 1995, 42, 756–762. [Google Scholar] [CrossRef]
- Wen, X.; Liu, H.; Zhang, L.; Zhang, J.; Fu, C.; Shi, X.; Chen, X.; Mijowska, E.; Chen, M.-J.; Wang, D.-Y. Large-scale converting waste coffee grounds into functional carbon materials as high-efficient adsorbent for organic dyes. Bioresour. Technol. 2019, 272, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Figueroa Campos, G.A.; Perez, J.P.H.; Block, I.; Sagu, S.T.; Saravia Celis, P.; Taubert, A.; Rawel, H.M. Preparation of Activated Carbons from Spent Coffee Grounds and Coffee Parchment and Assessment of Their Adsorbent Efficiency. Processes 2021, 9, 1396. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Cui, Z.; Fu, Z.; Yang, L.; Liu, G.; Li, M. Coffee grounds derived N enriched microporous activated carbons: Efficient adsorbent for post-combustion CO2 capture and conversion. J. Colloid Interface Sci. 2020, 578, 491–499. [Google Scholar] [CrossRef]
- Goncalves, M.; Castro, C.S.; Oliveira, L.C.A.; Carvalho, W.A. Green acid catalyst obtained from industrial wastes for glycerol etherification. Fuel Process. Technol. 2015, 138, 695–703. [Google Scholar] [CrossRef]
- Lykoudi, A.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of sulfamethoxazole with persulfate using spent coffee grounds biochar as activator. J. Environ. Manag. 2020, 271, 111022. [Google Scholar] [CrossRef]
- Agapay, R.C.; Liu, H.-C.; Ju, Y.-H.; Go, A.W.; Angkawijaya, A.E.; Nguyen, P.L.T.; Truong, C.T.; Quijote, K.L. Synthesis and Initial Evaluation of Solid Acid Catalyst Derived from Spent Coffee Grounds for the Esterification of Oleic Acid and Methanol. Waste Biomass Valorization 2021, 12, 4387–4397. [Google Scholar] [CrossRef]
- Luna-Lama, F.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Muñoz-Batista, M.J.; Caballero, A.; Balu, A.M.; Romero, A.A.; Luque, R. Non-porous carbonaceous materials derived from coffee waste grounds as highly sustainable anodes for lithium-ion batteries. J. Clean. Prod. 2019, 207, 411–417. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Li, H.; Zan, Y.; Li, X.; Zhu, Y.; Dou, M.; Wang, F. Sustainable Carbonaceous Materials Derived from Biomass as Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, 1805718. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Wen, X.; Chen, X.; Wen, Y.; Shi, X.; Mijowska, E. High yield conversion of biowaste coffee grounds into hierarchical porous carbon for superior capacitive energy storage. Sci. Rep. 2020, 10, 3518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wroniak, M.; Agnieszka, R. Innowacyjne Rozwiązania w Technologii Żywności i żywieniu Człowieka; Oddział Małopolski Polskiego Towarzystwa Technologów Żywności: Kraków, Poland, 2016. [Google Scholar]
- Geneau-Sbartaï, C.; Leyris, J.; Silvestre, F.; Rigal, L. Sunflower Cake as a Natural Composite: Composition and Plastic Properties. J. Agric. Food Chem. 2008, 56, 11198–11208. [Google Scholar] [CrossRef]
- Thinakaran, N.; Baskaralingam, P.; Pulikesi, M.; Panneerselvam, P.; Sivanesan, S. Removal of Acid Violet 17 from aqueous solutions by adsorption onto activated carbon prepared from sunflower seed hull. J. Hazard. Mater. 2008, 151, 316–322. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation and characterization of activated carbon from sunflower seed oil residue via microwave assisted K 2CO 3 activation. Bioresour. Technol. 2011, 102, 9794–9799. [Google Scholar] [CrossRef]
- Saleh, M.E.; El-Refaey, A.A.; Mahmoud, A.H. Effectiveness of sunflower seed husk biochar for removing copper ions from wastewater: A comparative study. Soil Water Res. 2016, 11, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Trytek, M.; Paduch, R.; Fiedurek, J.; Kandefer-Szerszeń, M. Monoterpeny-Stare związki, nowe zastosowania i biotechnologiczne metody ich otrzymywania. Biotechnologia 2007, 76, 135–155. [Google Scholar]
- Van Groenestijn, J.W.; Liu, J.X. Removal of alpha-pinene from gases using biofilters containing fungi. Atmos. Environ. 2002, 36, 5501–5508. [Google Scholar] [CrossRef]
- Sinhmar, P.S.; Gogate, P.R. Improved Activation of Titanium Dioxide Catalyst for Isomerization of Alpha Pinene and Understanding into Effect of Isomerization Parameters. Arab. J. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Michalkiewicz, B. The kinetics of homogeneous catalytic methane oxidation. Appl. Catal. A Gen. 2006, 307, 270–274. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics-a critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Ponomarev, D.; Mettee, H. Camphor and its Industrial Synthesis. Chem. Educ. J. 2016, 18, 1–4. [Google Scholar]
- Kapp, T.; Kammann, U.; Vobach, M.; Vetter, W. Synthesis of low and high chlorinated toxaphene and comparison of their toxicity by zebrafish (Danio rerio) embryo test. Environ. Toxicol. Chem. 2006, 25, 2884–2889. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Pájaro, E.; Villa, A.L.; Martínez-O, F. Selective synthesis of camphene from isomerization of α- and β-pinene over heterogeneous catalysts. Microporous Mesoporous Mater. 2021, 324, 111273. [Google Scholar] [CrossRef]
- Retajczyk, M.; Wróblewska, A.; Szymańska, A.; Michalkiewicz, B. Isomerization of limonene over natural zeolite-clinoptilolite. Clay Miner. 2019, 54, 121–129. [Google Scholar] [CrossRef]
- Młodzik, J.; Wróblewska, A.; Makuch, E.; Wróbel, R.J.; Michalkiewicz, B. Fe/EuroPh catalysts for limonene oxidation to 1,2-epoxylimonene, its diol, carveol, carvone and perillyl alcohol. Catal. Today 2016, 268, 111–120. [Google Scholar] [CrossRef]
- Wróblewska, A. The epoxidation of limonene over the ts-1 and ti-sba-15 catalysts. Molecules 2014, 19, 19907–19922. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef]
- Kumar, V.; Agarwal, A.K. a Review on Catalytic Terpene Transformation Over Heterogeneous Catalyst. Chem. Int. J. Curr. Res. Chem. Pharma. Sci. 2014, 1, 78–88. [Google Scholar]
- Ünveren, E.; Günüz, G.; Cakicioǧlu-Özkan, F. Isomerization of alpha-pinene over acid treated natural zeolite. Chem. Eng. Commun. 2005, 192, 386–404. [Google Scholar] [CrossRef] [Green Version]
- Miądlicki, P.; Wróblewska, A.; Kiełbasa, K.; Koren, Z.C.; Michalkiewicz, B. Sulfuric acid modified clinoptilolite as a solid green catalyst for solvent-free α-pinene isomerization process. Microporous Mesoporous Mater. 2021, 324, 111266. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Aho, A.; Ganbaatar, J.; Batsuren, D.; Utenkova, D.B.; Sen’kov, G.M.; Wärnå, J.; Murzin, D.Y.; Agabekov, V.E. Catalytic isomerization of A-pinene and 3-carene in the presence of modified layered aluminosilicates. Mol. Catal. 2017, 443, 193–202. [Google Scholar] [CrossRef]
- Zielinska, B.; Michalkiewicz, B.; Chen, X.; Mijowska, E.; Kalenczuk, R.J. Pd supported ordered mesoporous hollow carbon spheres (OMHCS) for hydrogen storage. Chem. Phys. Lett. 2016, 647, 14–19. [Google Scholar] [CrossRef]
- Akgül, M.; özyaĝci, B.; Karabakan, A. Evaluation of Fe- and Cr-containing clinoptilolite catalysts for the production of camphene from α-pinene. J. Ind. Eng. Chem. 2013, 19, 240–249. [Google Scholar] [CrossRef]
- Zou, J.-J.; Chang, N.; Zhang, X.; Wang, L. Isomerization and Dimerization of Pinene using Al-Incorporated MCM-41 Mesoporous Materials. ChemCatChem 2012, 4, 1289–1297. [Google Scholar] [CrossRef]
- Wróblewska, A.; Miądlicki, P.; Tołpa, J.; Sreńscek-Nazzal, J.; Koren, Z.C.; Michalkiewicz, B. Influence of the titanium content in the Ti-MCM-41 catalyst on the course of the α-pinene isomerization process. Catalysts 2019, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska, A.; Miądlicki, P.; Sreńscek-Nazzal, J.; Sadłowski, M.; Koren, Z.C.; Michalkiewicz, B. Alpha-pinene isomerization over Ti-SBA-15 catalysts obtained by the direct method: The influence of titanium content, temperature, catalyst amount and reaction time. Microporous Mesoporous Mater. 2018, 258, 72–82. [Google Scholar] [CrossRef]
- Lubkowski, K.; Arabczyk, W.; Grzmil, B.; Michalkiewicz, B.; Pattek-Janczyk, A. Passivation and oxidation of an ammonia iron catalyst. Appl. Catal. A Gen. 2007, 329, 137–147. [Google Scholar] [CrossRef]
- Launay, F.; Jarry, B.; Bonardet, J.L. Catalytic activity of mesoporous Ga-SBA-15 materials in α-pinene isomerisation: Similarities and differences with Al-SBA-15 analogues. Appl. Catal. A Gen. 2009, 368, 132–138. [Google Scholar] [CrossRef]
- Akpolat, O.; Gündüz, G.; Ozkan, F.; Beşün, N. Isomerization of α-pinene over calcined natural zeolites. Appl. Catal. A Gen. 2004, 265, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Grams, J.; Potrzebowska, N.; Goscianska, J.; Michalkiewicz, B.; Ruppert, A.M. Mesoporous silicas as supports for Ni catalyst used in cellulose conversion to hydrogen rich gas. Int. J. Hydrogen Energy 2016, 41, 8656–8667. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Gelves, J.F.; Márquez, M.A.; Dorkis, L.; Villa, A.L. Catalytic Isomerization of α-Pinene Epoxide Over a Natural Zeolite. Catal. Letters 2020, 150, 3132–3148. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Valverde, M.; Pérez, A.; Rico, J.L.; Mantilla, A.; Gómez, R. Synthesis of camphene by α-pinene isomerization using W2O3-Al2O3 catalysts. Top. Catal. 2010, 53, 1176–1178. [Google Scholar] [CrossRef]
- da Silva Rocha, K.A.; Robles-Dutenhefner, P.A.; Kozhevnikov, I.V.; Gusevskaya, E.V. Phosphotungstic heteropoly acid as efficient heterogeneous catalyst for solvent-free isomerization of α-pinene and longifolene. Appl. Catal. A Gen. 2009, 352, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, L.; Xie, C.X. Acidic functionalized ionic liquids as catalyst for the isomerization of α-pinene to camphene. Res. Chem. Intermed. 2016, 42, 559–569. [Google Scholar] [CrossRef]
- Wang, J.; Hua, W.; Yue, Y.; Gao, Z. MSU-S mesoporous materials: An efficient catalyst for isomerization of α-pinene. Bioresour. Technol. 2010, 101, 7224–7230. [Google Scholar] [CrossRef]
- Rabee, A.I.M.; Durndell, L.J.; Fouad, N.E.; Frattini, L.; Isaacs, M.A.; Lee, A.F.; Mekhemer, G.A.H.; do Santos, V.C.; Wilson, K.; Zaki, M.I. Citrate-mediated sol–gel synthesis of Al-substituted sulfated zirconia catalysts for α-pinene isomerization. Mol. Catal. 2018, 458, 206–212. [Google Scholar] [CrossRef]
- Zou, Z.; Tang, Y.; Jiang, C.; Zhang, J. Efficient adsorption of Cr(VI) on sunflower seed hull derived porous carbon. J. Environ. Chem. Eng. 2015, 3, 898–905. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shi, X.; Zhao, Q.; Li, Y.; Wang, J.; Yang, Y.; Bi, F.; Xu, J.; Liu, N. Defects controlled by acid-modulators and water molecules enabled UiO-67 for exceptional toluene uptakes: An experimental and theoretical study. Chem. Eng. J. 2022, 427, 131573. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, D.; Ji, W.; Bi, F.; Song, L.; Zhang, X. Uniform platinum nanoparticles loaded on Universitetet i Oslo-66 (UiO-66): Active and stable catalysts for gas toluene combustion. J. Colloid Interface Sci. 2022, 606, 1811–1822. [Google Scholar] [CrossRef]
- Serafin, J.; Kiełbasa, K.; Michalkiewicz, B. The new tailored nanoporous carbons from the common polypody (Polypodium vulgare): The role of textural properties for enhanced CO2 adsorption. Chem. Eng. J. 2022, 429, 131751. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Ng, E.P. The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Microporous Mesoporous Mater. 2014, 197, 316–323. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Textural and chemical properties of zinc chloride activated carbons prepared from pistachio-nut shells. Mater. Chem. Phys. 2006, 100, 438–444. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Jiang, Z.; Shi, M.; Sheng, R.; Liu, Z.; Zhang, S.; Cao, Y.; Wei, T.; Fan, Z. Large-surface-area activated carbon with high density by electrostatic densification for supercapacitor electrodes. Carbon N. Y. 2021, 175, 281–288. [Google Scholar] [CrossRef]
- Martínez-Casillas, D.C.; Mascorro-Gutiérrez, I.; Arreola-Ramos, C.E.; Villafán-Vidales, H.I.; Arancibia-Bulnes, C.A.; Ramos-Sánchez, V.H.; Cuentas-Gallegos, A.K. A sustainable approach to produce activated carbons from pecan nutshell waste for environmentally friendly supercapacitors. Carbon N. Y. 2019, 148, 403–412. [Google Scholar] [CrossRef]
- Pagalan, E.; Sebron, M.; Gomez, S.; Salva, S.J.; Ampusta, R.; Macarayo, A.J.; Joyno, C.; Ido, A.; Arazo, R. Activated carbon from spent coffee grounds as an adsorbent for treatment of water contaminated by aniline yellow dye. Ind. Crops Prod. 2020, 145, 111953. [Google Scholar] [CrossRef]
- Gęsikiewicz-Puchalska, A.; Zgrzebnicki, M.; Michalkiewicz, B.; Narkiewicz, U.; Morawski, A.W.; Wrobel, R.J. Improvement of CO2 uptake of activated carbons by treatment with mineral acids. Chem. Eng. J. 2017, 309, 159–171. [Google Scholar] [CrossRef]
- Wijayati, N.; Pranowo, H.D.; Jumina, T.; Chuah, G.K. Characterization of ZHY and TCA/ZHY Catalysts for Hydration of α-Pinene. Int. J. Chem. Eng. Appl. 2013, 4, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, B.; Wróblewska, A.; Maślana, K.; Miądlicki, P.; Kiełbasa, K.; Rozmysłowska-Wojciechowska, A.; Petrus, M.; Woźniak, J.; Jastrzębska, A.M.; Michalkiewicz, B.; et al. High catalytic performance of 2D Ti3C2Tx MXene in α-pinene isomerization to camphene. Appl. Catal. A Gen. 2020, 604, 117765. [Google Scholar] [CrossRef]
- Allahverdiev, A.I.; Irandoust, S.; Murzin, D.Y. Isomerization of α-pinene over clinoptilolite. J. Catal. 1999, 185, 352–362. [Google Scholar] [CrossRef]
















| Catalyst | Conversion of Alpha-Pinene (mol.%) | Selectivity of Camphene (mol.%) | Selectivity of Limonene (mol.%) | Ref. |
|---|---|---|---|---|
| HCl-modified clinoptilolite | 41 | 57 | 32 | [65] |
| SO4/AlxZrO2 | 32 | ¯ | ¯ | [82] |
| W2O3–Al2O3 | 73 | 55 | ¯ | [78] |
| Fe-loaded clinoptilolite | 100 | 38 | 2 | [69] |
| Cr-loaded clinoptilolite | 100 | 24 | 11 | [69] |
| H2SO4-modified clinoptilolite from Turkey | 18 | 46 | 19 | [66] |
| Calcined natural zeolites | 100 | 32 | 25 | [75] |
| Al-MCM-41 | 98 | 30 | 30 | [70] |
| Ti-MCM-41 | 98 | 35 | 21 | [71] |
| Ti-SBA-15 | 98 | 24 | 24 | [72] |
| Activated carbon from coffee grounds | 84 | 34 | 38 | In this study |
| Sample | SBET (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | V0.73–1 nm (cm3/g) | V1–2 nm (cm3/g) | Acid-Site Concentration (mmol/g) |
|---|---|---|---|---|---|---|
| O_AC | 1416 | 0.643 | 0.482 | 0.097 | 0.154 | 0.25 |
| C_AC | 1566 | 0.694 | 0.540 | 0.123 | 0.139 | 0.50 |
| S_AC | 1366 | 0.584 | 0.477 | 0.097 | 0.111 | 0.45 |
| Assignment | O_AC | C_AC | S_AC |
|---|---|---|---|
| C | 62.6 | 62.7 | 63.6 |
| C–O | 13.0 | 12.9 | 11.7 |
| Keto-enolic | 1.6 | 2.2 | 2.8 |
| C=O | 5.9 | 5.9 | 6.3 |
| COOH | 2.4 | 2.5 | 2.7 |
| Satellite | 14.6 | 13.9 | 12.8 |
| Sample | O 1s | C 1s | Si 2p |
|---|---|---|---|
| O_AC | 7.43 | 91.49 | 1.08 |
| C_AC | 5.89 | 93.78 | 0.33 |
| S_AC | 6.6 | 93.4 | 0 |
| Sample | Wt.% | ||||
|---|---|---|---|---|---|
| Si | S | Cl | K | Ca | |
| O_AC | 0.264 | 0.416 | 0.182 | 0.595 | 0.289 |
| C_AC | 0.268 | 0.244 | 0.696 | 0.997 | 0.000 |
| S_AC | 0.246 | 0.418 | 0.155 | 0.225 | 0.280 |
| Sample | k (h−1) | R2 |
|---|---|---|
| O_AC | 0.3178 | 0.9309 |
| C_AC | 0.3311 | 0.9670 |
| S_AC | 0.0573 | 0.9049 |
| No. | Steps | α-Pinene = Tricyclene | α-Pinene = Camphene | α-Pinene = Limonene | α-Pinene = α+γ-Terpinene | α-Pinene = Terpinolene | Limonene = p-Cymene | α+γ-Terpinene = p-Cymene | Terpinolene = p-Cymene |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Z + A Ξ Z·(A) | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 2 | Z·(A) ⇒ Z·(A)1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | Z·(A) ⇒ Z·(A)2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 4 | Z·(A)1 ⇔ Z·(B) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | Z·(A)1 ⇔ Z·(C) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | Z·(B) Ξ Z + B | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | Z·(C) Ξ Z + C | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | Z·(A)2 ⇒ Z·(D) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 9 | Z·(D) Ξ Z + D | 0 | 0 | 1 | 0 | 0 | −1 | 0 | 0 |
| 10 | Z·(A)2 ⇒ Z·(E) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 11 | Z·(E) Ξ Z + (E) | 0 | 0 | 0 | 1 | 0 | 0 | −1 | 0 |
| 12 | Z·(A)2 ⇒ Z·(F) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 13 | Z·(F) Ξ Z + F | 0 | 0 | 0 | 0 | 1 | 0 | 0 | −1 |
| 14 | Z·(D) ⇒ Z·(G) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 15 | Z·(E) ⇒ Z·(G) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 16 | Z·(F) ⇒ Z·(G) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 17 | Z·(G) Ξ G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Dimensionless Parameter | Estimated Value | Standard Error (±) |
|---|---|---|
| f1 | 0.04 | 0.0036 |
| f2 | 0.05 | 0.0011 |
| f3 | 0.08 | 0.0041 |
| f4 | 0.38 | 0.0278 |
| f5 | 0.57 | 0.0294 |
| f6 | 0.19 | 0.0165 |
| f7 | 0.05 | 0.0077 |
| f8 | 0.05 | 0.0082 |
| f9 | 0.04 | 0.0063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, A.; Miądlicki, P.; Kiełbasa, K.; Kujbida, M.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Wróblewska, A. Activated Carbons Obtained from Orange Peels, Coffee Grounds, and Sunflower Husks—Comparison of Physicochemical Properties and Activity in the Alpha-Pinene Isomerization Process. Materials 2021, 14, 7448. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14237448
Kamińska A, Miądlicki P, Kiełbasa K, Kujbida M, Sreńscek-Nazzal J, Wróbel RJ, Wróblewska A. Activated Carbons Obtained from Orange Peels, Coffee Grounds, and Sunflower Husks—Comparison of Physicochemical Properties and Activity in the Alpha-Pinene Isomerization Process. Materials. 2021; 14(23):7448. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14237448
Chicago/Turabian StyleKamińska, Adrianna, Piotr Miądlicki, Karolina Kiełbasa, Marcin Kujbida, Joanna Sreńscek-Nazzal, Rafał Jan Wróbel, and Agnieszka Wróblewska. 2021. "Activated Carbons Obtained from Orange Peels, Coffee Grounds, and Sunflower Husks—Comparison of Physicochemical Properties and Activity in the Alpha-Pinene Isomerization Process" Materials 14, no. 23: 7448. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14237448