FeCl3-Modified Carbonaceous Catalysts from Orange Peel for Solvent-Free Alpha-Pinene Oxidation
Abstract
:1. Introduction
2. Materials and Methods
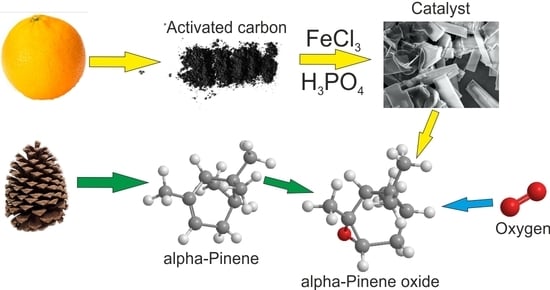
2.1. Preparation Activated Carbon (AC)
2.2. Preparation of Metallic Catalysts
2.3. Characterizing the Catalysts Obtained from Biomass
2.4. Alpha-Pinene Oxidation Method
3. Results and Discussion
3.1. Characterization of the Obtained Catalysts
3.2. Activity of the Obtained Modified Carbonaceous Catalysts
3.3. Determination of the Kinetics Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadirvelu, K.; Namasivayam, C. Activated carbon from coconut coirpith as metal adsorbent: Adsorption of Cd(II) from aqueous solution. Adv. Environ. Res. 2003, 7, 471–478. [Google Scholar] [CrossRef]
- Malik, R.; Ramteke, D.; Wate, S. Adsorption of malachite green on groundnut shell waste based powdered activated carbon. Waste Manag. 2007, 27, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Artola, A.; Balaguer, M.D.; Rigola, M. Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. Chem. Eng. J. 2003, 94, 231–239. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Grams, J.; Potrzebowska, N.; Goscianska, J.; Michalkiewicz, B.; Ruppert, A.M. Mesoporous silicas as supports for Ni catalyst used in cellulose conversion to hydrogen rich gas. Int. J. Hydrogen Energy 2016, 41, 8656–8667. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged Citric Acid Fermentation on Orange Peel Autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef]
- López, J.; Ángel, S.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef]
- Michalkiewicz, B. The kinetics of homogeneous catalytic methane oxidation. Appl. Catal. A Gen. 2006, 307, 270–274. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crop. Prod. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Ahmed, S.; Rafat, M.; Ahmed, A. Nitrogen doped activated carbon derived from orange peel for supercapacitor application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035008. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Chen, Z.; Cheng, Y.; Wang, X.; Yang, X.; Wang, Z. Preparation and electrochemical performance of orange peel based-activated carbons activated by different activators. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 221–227. [Google Scholar] [CrossRef]
- Arie, A.A.; Kristianto, H.; Suharto, I.; Halim, M.; Lee, J.K. Preparation of Orange Peel Based Activated Carbons as Cathodes in Lithium Ion Capacitors. Adv. Mater. Res. 2014, 896, 95–99. [Google Scholar] [CrossRef]
- Shukla, S.K.; Al Mushaiqri, N.R.S.; Al Subhi, H.M.; Yoo, K.; Al Sadeq, H. Low-cost activated carbon production from organic waste and its utilization for wastewater treatment. Appl. Water Sci. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Chen, D.; Liu, J.; Zhang, Y.; Chen, J.; Du, C.; Xie, M. Facile preparation of porous carbons derived from orange peel via basic copper carbonate activation for supercapacitors. J. Alloy. Compd. 2020, 823, 153747. [Google Scholar] [CrossRef]
- Lubkowski, K.; Arabczyk, W.; Grzmil, B.; Michalkiewicz, B.; Pattek-Janczyk, A. Passivation and oxidation of an ammonia iron catalyst. Appl. Catal. A: Gen. 2007, 329, 137–147. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Pirajan, J.C. Activated Carbon Prepared from Orange Peels Coated with Titanium Oxide Nanoparticles: Characterization and Applications in the Decomposition of NOx. Orient. J. Chem. 2014, 30, 451–461. [Google Scholar] [CrossRef]
- Arcibar-Orozco, J.A.; Rangel-Mendez, J.R.; Bandosz, T.J. Reactive adsorption of SO2 on activated carbons with deposited iron nanoparticles. J. Hazard. Mater. 2013, 246–247, 300–309. [Google Scholar] [CrossRef]
- Veerakumar, P.; Veeramani, V.; Chen, S.-M.; Madhu, R.; Liu, S.-B. Palladium Nanoparticle Incorporated Porous Activated Carbon: Electrochemical Detection of Toxic Metal Ions. ACS Appl. Mater. Interfaces 2016, 8, 1319–1326. [Google Scholar] [CrossRef]
- Gupta, V.; Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar] [CrossRef]
- Granström, K.M. Wood processing as a source of terpene emissions compared to natural sources. In Proceedings of the WIT Transactions on Ecology and the Environment, Cambridge, UK, 2–5 July 2007; WIT Press: Southampton, UK, 2007; Volume 101, pp. 263–272. [Google Scholar]
- Polo, C.M.; Moraes, T.M.; Pellizzon, C.; Marques, M.; da Rocha, L.R.M.; Hiruma-Lima, C.A. Gastric Ulcers in Middle-Aged Rats: The Healing Effect of Essential Oil fromCitrus aurantiumL. (Rutaceae). Evid.-Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santin, J.R.; Lemos, M.; Klein-Júnior, L.C.; Machado, I.D.; Costa, P.; de Oliveira, A.P.; Tilia, C.; de Souza, J.P.; de Sousa, J.P.B.; Bastos, J.K.; et al. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn-Schmiedebergs Arch. fur Exp. Pathol. Pharmakol. 2010, 383, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Fernandes, H.; Piauilino, C.; Pereira, S.; Carvalho, K.; Chaves, M.; Soares, P.; Miura, L.; Leite, J.; Oliveira, R.; et al. Gastroprotective activity of Zanthoxylum rhoifolium Lam. in animal models. J. Ethnopharmacol. 2011, 137, 700–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozza, A.L.; Pellizzon, C.H. Essential oils from medicinal and aromatic plants: A review of the gastroprotective and ulcer-healing activities. Fundam. Clin. Pharmacol. 2012, 27, 51–63. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Pinheiro, M.D.A.; Magalhães, R.M.; Torres, D.M.; Cavalcante, R.C.; Mota, F.S.X.; Coelho, E.A.O.; Moreira, H.P.; Lima, G.C.; Araújo, P.C.; et al. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn. Mag. 2015, 11, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
- Linares, D.; Fontanille, P.; Larroche, C. Exploration of α-pinene degradation pathway of Pseudomonas rhodesiae CIP 107491. Application to novalic acid production in a bioreactor. Food Res. Int. 2009, 42, 461–469. [Google Scholar] [CrossRef]
- Kucharska, M.; Szymańska, J.A.; Wesołowski, W.; Bruchajzer, E.; Frydrych, B. Comparison of chemical composition of selected essential oils used in respiratory diseases. Med. Pr. 2017, 69, 167–178. [Google Scholar] [CrossRef]
- Encinar, J.M.; Beltrán, F.J.; Frades, J.M. Liquid phase oxidation of α-pinene initiated by ozone. 2: Formation of verbenol, verbenone and acid products. Chem. Eng. Technol. 1994, 17, 187–194. [Google Scholar] [CrossRef]
- Wender, P.A.; Badham, N.F.; Conway, S.P.; Floreancig, P.E.; Glass, T.E.; Gränicher, C.; Houze, J.B.; Jänichen, J.; Lee, D.; Marquess, D.G.; et al. The Pinene Path to Taxanes. 5. Stereocontrolled Synthesis of a Versatile Taxane Precursor. J. Am. Chem. Soc. 1997, 119, 2755–2756. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Das, B.K.; Clark, J.H. Enhanced selectivity in green catalytic epoxidation using a supported cobalt complex. Green Chem. 2007, 9, 845–848. [Google Scholar] [CrossRef]
- Cánepa, A.L.; Chanquía, C.M.; Vaschetti, V.M.; Eimer, G.A.; Casuscelli, S.G. Biomass toward fine chemical products: Oxidation of α-pinene over sieves nanostructured modified with vanadium. J. Mol. Catal. A Chem. 2015, 404–405, 65–73. [Google Scholar] [CrossRef]
- Casuscelli, S.G.; Eimer, G.A.; Canepa, A.; Heredia, A.C.; Poncio, C.E.; Crivello, M.E.; Perez, C.F.; Aguilar, A.; Herrero, E.R. Ti-MCM-41 as catalyst for α-pinene oxidation: Study of the effect of Ti content and H2O2 addition on activity and selectivity. Catal. Today 2008, 133–135, 678–683. [Google Scholar] [CrossRef]
- On, D.T.; Kapoor, M.; Joshi, P.; Bonneviot, L.; Kaliaguine, S. Catalytic epoxidation of α-pinene over bifunctional mesoporous molecular sieves. Catal. Lett. 1997, 44, 171–176. [Google Scholar] [CrossRef]
- Suh, Y.-W.; Kim, N.-K.; Ahn, W.-S.; Rhee, H.-K. One-pot synthesis of campholenic aldehyde from α-pinene over Ti-HMS catalyst II: Effects of reaction conditions. J. Mol. Catal. A Chem. 2003, 198, 309–316. [Google Scholar] [CrossRef]
- Becerra, J.-A.; González, L.-M.; Villa, A.-L. Kinetic study of α-pinene allylic oxidation over FePcCl16-NH2-SiO2 catalyst. J. Mol. Catal. A Chem. 2016, 423, 12–21. [Google Scholar] [CrossRef]
- Maksimchuk, N.; Melgunov, M.; Mrowiecbialon, J.; Jarzebski, A.; Kholdeeva, O. H2O2-based allylic oxidation of α-pinene over different single site catalysts. J. Catal. 2005, 235, 175–183. [Google Scholar] [CrossRef]
- Aberkouks, A.; Mekkaoui, A.A.; Boualy, B.; EL Houssame, S.; Ali, M.A.; El Firdoussi, L. Co-Ag supported ZnO: An efficient and recyclable heterogeneous catalyst for the oxidation of natural terpenes. Mater. Today Proc. 2019, 13, 453–457. [Google Scholar] [CrossRef]
- Mal, N.K.; Bhaumik, A.; Matsukata, M.; Fujiwara, M. Syntheses of Mesoporous Hybrid Iron Oxophenyl Phosphate, Iron Oxophosphate, and Sulfonated Oxophenyl Phosphate. Ind. Eng. Chem. Res. 2006, 45, 7748–7751. [Google Scholar] [CrossRef]
- Li, G.; Zhong, L.; Yuan, X.; Wu, J.; Luo, H.-A. Preparation, Characterization and Catalytic Properties of Sn-Containing MCM-41. J. Inorg. Mater. 2010, 25, 1041–1046. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, H.; Hibino, M.; Honma, I. Synthesis of Hexagonal Mesostructured FePO4 Using Cationic Surfactant as the Template. Chem. Lett. 2004, 33, 774–775. [Google Scholar] [CrossRef]
- Cao, F.; Li, D. Biotemplate synthesis of monodispersed iron phosphate hollow microspheres. Bioinspir. Biomim. 2010, 5, 16005. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Xie, K.; Li, J.; Chen, Y.; Tang, Y.; Zhou, Y.; Lu, T. Synthesis and characterization of multi–wall carbon nanotubes supported-hydrated iron phosphate cathode material for lithium–ion cells by a novel homogeneous precipitation method. Ionics 2012, 18, 721–729. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Liu, J.; Cheng, Z.; Si, D.; Geng, B. Kinetic manipulation of the morphology evolution of FePO4 microcrystals: From rugbies to porous microspheres. CrystEngComm 2009, 11, 2510–2515. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.C.; Borba, C.E.; Godinho, M.; Perondi, D.; Schontag, J.M.; Wenzel, B.M. Phosphorus adsorption in Fe-loaded activated carbon: Two-site monolayer equilibrium model and phenomenological kinetic description. Chem. Eng. J. 2019, 361, 751–763. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, H.; Yan, Y.; Zhang, X. Preparation and characterization of porous Fe-Cu mixed oxides modified ZSM-5 coating/PSSF for continuous degradation of phenol wastewater. Microporous Mesoporous Mater. 2017, 240, 108–116. [Google Scholar] [CrossRef]
- Yuan, M.; Deng, W.; Dong, S.; Li, Q.; Zhao, B.; Su, Y. Montmorillonite based porous clay heterostructures modified with Fe as catalysts for selective catalytic reduction of NO with propylene. Chem. Eng. J. 2018, 353, 839–848. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, W.; Yao, Y. Preparation of Nanoscale Iron (III) Phosphate by Using Ferro-Phosphorus as Raw Material. IOP Conf. Series Earth Environ. Sci. 2019, 252, 022032. [Google Scholar] [CrossRef]
- Viswanathan, B.; Murugesan, S.; Ariharan, A.; Lakhi, K.S. Hetero Atom Substituted Carbon—Potential Hydrogen Storage Materials. Adv. Porous Mater. 2013, 1, 122–128. [Google Scholar] [CrossRef]
- Jia, Y.; Luo, T.; Yu, X.-Y.; Sun, B.; Liu, J.-H.; Huang, X.-J. Synthesis of monodispersed α-FeOOH nanorods with a high content of surface hydroxyl groups and enhanced ion-exchange properties towards As(v). RSC Adv. 2013, 3, 15805–15811. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; You, Y.; Zheng, X.; Wen, J. Synthesis of different structured FePO4 for the enhanced conversion of methyl cellulose to 5-hydroxymethylfurfural. RSC Adv. 2017, 7, 51281–51289. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Sun, Y.; Jia, X. Hydrothermal synthesis of iron phosphate microspheres constructed by mesoporous polyhedral nanocrystals. Mater. Charact. 2015, 107, 182–188. [Google Scholar] [CrossRef]
- Benaddi, H.; Legras, D.; Rouzaud, J.; Beguin, F. Influence of the atmosphere in the chemical activation of wood by phosphoric acid. Carbon 1998, 36, 306–309. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Huang, M.; Ma, H.-L.; Zhang, Z.-Q.; Gao, J.-M.; Zhu, Y.-L.; Han, X.-J.; Guo, X.-Y. Preparation of a Carbon-Based Solid Acid Catalyst by Sulfonating Activated Carbon in a Chemical Reduction Process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Xue, Y.; Zhang, K.; Zhang, Y. Synthesis of FePO4·2H2O nanoplates and their usage for fabricating superior high-rate performance LiFePO4. Electrochim. Acta 2011, 56, 4294–4298. [Google Scholar] [CrossRef]
- Masquelier, C.; Reale, P.; Wurm, C.; Morcrette, M.; Dupont, L.; Larcher, D. Hydrated Iron Phosphates FePO4 ⋅ nH2O and Fe4( P2O7)3 ⋅ nH2O as 3 V Positive Electrodes in Rechargeable Lithium Batteries. J. Electrochem. Soc. 2002, 149, A1037–A1044. [Google Scholar] [CrossRef]
- Gęsikiewicz-Puchalska, A.; Zgrzebnicki, M.; Michalkiewicz, B.; Narkiewicz, U.; Morawski, A.; Wrobel, R. Improvement of CO2 uptake of activated carbons by treatment with mineral acids. Chem. Eng. J. 2017, 309, 159–171. [Google Scholar] [CrossRef]
- Pramanik, M.; Imura, M.; Lin, J.; Kim, J.; Kim, J.H.; Yamauchi, Y. Shape-controlled synthesis of mesoporous iron phosphate materials with crystallized frameworks. Chem. Commun. 2015, 51, 13806–13809. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Robles-Dutenhefner, P.; Menini, L.; Gusevskaya, E.V. Cobalt catalyzed autoxidation of monoterpenes in acetic acid and acetonitrile solutions. J. Mol. Catal. A Chem. 2003, 201, 71–77. [Google Scholar] [CrossRef]
- Ancel, J.; Maksimchuk, N.; Simakova, I.; Semikolenov, V. Kinetic peculiarities of α-pinene oxidation by molecular oxygen. Appl. Catal. A Gen. 2004, 272, 109–114. [Google Scholar] [CrossRef]
- Emanuel, N.M.; Knorre, D.G. Kurs Khimicheskoi Kinetiki (Course in Chemical Kinetics); Vysshaya Shkola: Moscow, Russia, 1962. [Google Scholar]










| Catalyst | Main Products | Selectivity to Alpha-Pinene Oxide (mol%) | Conversion of Alpha-Pinene (mol%) | Solvent/Oxidant | Ref. |
|---|---|---|---|---|---|
| V-MCM-41 * | Verbenone, trans-Sobrerol, Campholenic aldehyde | 5 | 13 | Acetonitrile/H2O2 | [34] |
| Ti-MCM-41 * | Verbenone, Verbenol, Campholenic aldehyde | 27 | 39 | Acetonitrile/H2O2 | [35] |
| MCM-41 * and HMS ** containing metal ions | Alpha-pinene oxide, 1,2-pinane diol | 100 | 11 | Chloroform/ TBHP *** or H2O2 | [36] |
| Ti-HMS ** | Verbenone, Verbenol, Campholenic aldehyde | 13 | 30 | Acetonitrile/ TBHP *** | [37] |
| FePcCl16-NH2-SiO2 | Verbenone | 16 | 61 | Acetone/TBHP *** | [38] |
| H5PW11TiO40/silica | Verbenone, Verbenol | - | 60 | Acetonitrile/H2O2 | [39] |
| Co-Ag supported ZnO | Verbenone, Myrtenal | - | 100 | Acetonitrile/H2O2 | [40] |
| FeCl3-modified carbonaceous catalysts | Alpha-pinene oxide, Verbenone, Verbenol | 35 | 40 | Absent/O2 | In this work |
| Sample | SBET (m2/g) | Vtot (cm3/g) | Fe (wt%) |
|---|---|---|---|
| O_Fe3_H3PO4 | 221 | 0.132 | 25.01 |
| O_Fe6_H3PO4 | 602 | 0.296 | 17.94 |
| O_Fe9_H3PO4 | 1300 | 0.608 | 6.12 |
| Assignment | O_Fe3_H3PO4 | O_Fe6_H3PO4 | O_Fe9_H3PO4 |
|---|---|---|---|
| C | 46.7 | 50.8 | 61.9 |
| C–O | 22.4 | 23.7 | 11.4 |
| Keto-enolic | 0.0 | 0.0 | 3.0 |
| C=O | 10.2 | 10.6 | 5.9 |
| COOH | 6.0 | 6.6 | 2.9 |
| Satellite | 14.7 | 8.3 | 14.9 |
| Sample | At. % | |||
|---|---|---|---|---|
| C | O | Fe | P | |
| O_Fe3_H3O4 | 28.8 | 45.6 | 13.1 | 12.5 |
| O_Fe6_ H3O4 | 53.2 | 30.9 | 8.4 | 7.6 |
| O_Fe9_H3PO4 | 89.8 | 9.2 | 1.0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, A.; Miądlicki, P.; Kiełbasa, K.; Serafin, J.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Wróblewska, A. FeCl3-Modified Carbonaceous Catalysts from Orange Peel for Solvent-Free Alpha-Pinene Oxidation. Materials 2021, 14, 7729. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14247729
Kamińska A, Miądlicki P, Kiełbasa K, Serafin J, Sreńscek-Nazzal J, Wróbel RJ, Wróblewska A. FeCl3-Modified Carbonaceous Catalysts from Orange Peel for Solvent-Free Alpha-Pinene Oxidation. Materials. 2021; 14(24):7729. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14247729
Chicago/Turabian StyleKamińska, Adrianna, Piotr Miądlicki, Karolina Kiełbasa, Jarosław Serafin, Joanna Sreńscek-Nazzal, Rafał Jan Wróbel, and Agnieszka Wróblewska. 2021. "FeCl3-Modified Carbonaceous Catalysts from Orange Peel for Solvent-Free Alpha-Pinene Oxidation" Materials 14, no. 24: 7729. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14247729