Comparative Analysis of Lead Removal from Liquid Copper by ICF and CCF Refining Technologies
Abstract
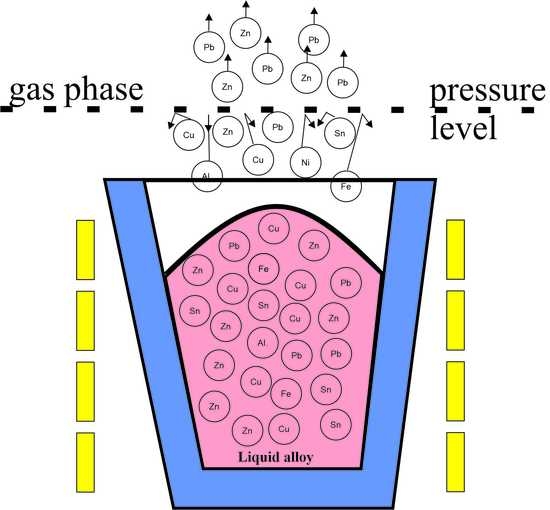
:1. Introduction
2. State of the Art
3. Materials and Methods
3.1. Materials Used in the Research
3.2. Research Apparatus
3.3. Research Methodology and Parameters
- βl—the mass transfer coefficient of Pb in liquid phase, m·s−1,
- βg—the mass transfer coefficient of Pb in gas phase, m·s−1,
- ke—the evaporation rate coefficient, m·s−1.
- —the lead concentration in copper: initial and at time t, wt. %,
- F—the evaporation surface, m2,
- V—the metal volume, m3,
- (t − t0)—the duration of the process, s.
- νm—the surface velocity of the inductively mixed liquid metal, m s−1,
- rm—the radius of the surface of the liquid metal (most often taken as the inner radius of the crucible), m,
- DPb—the lead diffusion coefficient in liquid copper, m2 s−1,
- α—the evaporation constant,
- p0Pb—the equilibrium vapor pressure of lead over the liquid alloy, Pa,
- γPb—the activity factor of lead in liquid copper,
- MCu, MPb—the molar masses, respectively, of copper and lead, g mol−1,
- ρCu—the density of copper, g m−3.
4. Results and Discussion
- Lead transport from inside liquid copper to the surface;
- The phenomenon of evaporation from the surface of liquid copper;
- The transport of lead vapor into the gas phase.
- Creating photos of molten metal using a fast camera;
- Establishing the geometry of the created meniscus based on these photos;
- Charting a function graph that describes the meniscus during different parameters of the process;
- Estimating the surface area using Wolfram Mathematica and MicroStation software.
4.1. Mass Transfer in the Liquid Phase
4.2. The Process of Evaporation from the Interface
5. Conclusions
- The process of refining copper from lead conducted in vacuum induction furnaces happens faster inside an aggregate with a cold crucible, as compared to a typical crucible furnace. This is caused by a significant difference in the size of the metal bath’s surface area. The calculated surface areas of the meniscus formed during smelting alloys using a furnace with a cold crucible are significantly larger compared to those where the inserts were melted in induction crucible furnaces.
- For the experiments conducted inside an induction crucible furnace, lowering the working pressure inside the furnace chamber from 1000 to 10 Pa while increasing the temperature from 1323 to 1473 K was accompanied by a drop in the lead concentration inside the alloy of between 69 and 96%, compared to its initial mass. At the same time, the value of the overall mass transport coefficient kPb increased from 3.50 × 10−5 to 5.27 × 10−5 ms−1.
- For the experiments conducted inside a cold crucible furnace, approximate values of lead removal appeared for lower temperatures (1273 to 1323 K), confirming that the analyzed process happens faster in this aggregate.
- When the pressure is lowered, the rate-determining step of the analyzed process changes. When the pressure is equal to 10 Pa, the value of resistance related to mass transport in the liquid phase (Rl) and the reaction on the surface of the liquid metal (Re) comprises nearly 60% of the overall process resistance.
- For the experiments conducted inside a cold crucible surface, in the overall temperatures and pressures used, the rate of the process is limited by mass transport in the gas phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tetsui, T.; Kobayashi, T.; Ueno, T.; Harada, H. Consideration of the influence of contamination from oxide crucibles on TiAl cast material, and the possibility of achieving low-purity TiAl precision cast turbine wheels. Intermetallics 2012, 31, 274–281. [Google Scholar] [CrossRef]
- Zhang, H.R.; Tang, X.X.; Zhou, L.; Gao, M.; Zhou, C.G.; Zhang, H. Interactions between Ni-44Ti-5Al-2Nb-Mo alloy and oxide ceramics during directional solidification process. J. Mater. Sci. 2012, 47, 6451–6458. [Google Scholar] [CrossRef]
- Schafföner, S.; Aneziris, C.G.; Berek, H.; Hubálková, J.; Rotmann, B.; Friedrich, B. Corrosion behavior of calcium zirconate refractories in contact with titanium aluminide melts. J. Eur. Ceram. Soc. 2015, 35, 1097–1106. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Mandil, G.; Le, V.T.; Paris, H.; Suard, M. Building new entities from existing titanium part by electron beam melting: Microstructures and mechanical properties. Int. J. Adv. Manuf. Technol. 2015, 85, 1835–1846. [Google Scholar] [CrossRef] [Green Version]
- Rabadia, C.D.; Liu, Y.J.; Wang, L.; Suna, H.; Zhang, L.C. Laves phase precipitation in Ti-Zr-Fe-Cr alloys with high strength and large plasticity. Mater. Des. 2018, 154, 228–238. [Google Scholar] [CrossRef]
- Baudana, G.; Biamino, S.; Klöden, B.; Kirchner, A.; Weißgärber, T.; Kieback, B.; Pavese, M.; Ugues, D.; Fino, P.; Badini, C. Electron beam melting of Ti-48Al-Nb-0.7Cr- 0.3Si: Feasibility investigation. Intermetallics 2016, 73, 43–49. [Google Scholar] [CrossRef]
- Güther, V.; Keitel, H.; Klose, J.; Rothe, C.; Eulitz, I. Development of an Industrial Recycling Technology for TiAl Revert. In Proceedings of the 5th International Workshop on Titanium Aluminides, Tokyo, Japan, 28 August–2 September 2016. [Google Scholar]
- Kamyshnykova, K.; Lapin, J. Vacuum induction melting and solidification of TiAl-based alloy in graphite crucibles. Vacuum 2018, 154, 218–226. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Wu, Y.; Wang, X.; Hu, R. A combined electromagnetic levitation melting, counter gravity casting, and mold preheating furnace for producing TiAl alloy. Adv. Eng. Mater. 2017, 20, 1–7. [Google Scholar] [CrossRef]
- Kim, G.; Kvyatkovskii, A. At all. Vacuum Processing of Crude Copper. Trudy Altaisk Gorno-Met-Nauczno-Isled. Inst. 1963, 13, 86–89. [Google Scholar]
- Kametani, H.C.; Yamauchi, C.Y. A Fundamental Study on Vacuum Lift Refining of Molten Copper. Trans. Jpn. Inst. Met. 1972, 13, 13–20. [Google Scholar] [CrossRef]
- Kametani, H.C.; Yamauchi, C.Y.; Murao, K.; Hayashida, M. A Fundamental Study of Vacuum Treatment of Molten Matte and White Metal. Trans. JIM 1973, 14, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Komorova, L. Odstranovanie necistot z medi vakuovou rafinaciou. Hutnicke Listy 1973, 8, 577–582. [Google Scholar]
- Ohno, R. Rates of Evaporation of Silver, Lead, and Bismuth from Copper Alloys in Vacuum Induction Melting. Metall. Trans. B 1976, 7B, 647–653. [Google Scholar] [CrossRef]
- Ohno, R. Rates of evaporation of silver, lead, bismuth, and sulphur from molten copper alloys stirred at different speeds under reduced pressure. Trans. JIM 1977, 18, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Ozberk, B.; Guthrie, R.I.L. Evaluation of Vacuum Induction Melting for Copper Refining. Trans. Inst. Min. Met. Sect. C 1985, 94, 146–157. [Google Scholar]
- Ozberk, B.; Guthrie, R.I.L. Kinetic Model for the Vacuum Refining Inductively Stirred Copper. Trans. Melts, Metallurgical Transaction B 1986, 17B, 87–103. [Google Scholar] [CrossRef]
- Blacha, L. Bleientfernung aus Kupferlegierungen im Prozess der Vakuumraffination. Arch. Metall. 2003, 48, 105–127. [Google Scholar]
- Blacha, L. Eliminacja Ołowiu i Antymonu z Miedzi i jej Stopów w Procesie Rafinacji Próżniowej; Wydawnictwo Pol. Śl.: Gliwice, Poland, 2001. [Google Scholar]
- Smalcerz, A.; Blacha, L. Removal of lead from blister copper by melting in the induction vacuum furnace. Arch. Foundry Eng. 2020, 20, 84–88. [Google Scholar]
- Siwiec, G.; Buliński, P.; Palacz, M.; Smołka, J.; Blacha, L. Investigation on the process of lead removal from Cu-Pb alloys during their melting in vacuum induction furnace. Arch. Metall. Mater. 2017, 62, 2449–2453. [Google Scholar] [CrossRef] [Green Version]
- Machlin, E.S. Kinetics of Vacuum Induction Refining-Theory; The American Institute of Mining, Metallurgical, and Petroleum Engineers: New York, NY, USA, 1960; pp. 314–326. [Google Scholar]
- Evans, R.; Greenwood, D. Liquid metals, 1976: Invited and contributed papers from the third. In Proceedings of the Third International Conference on Liquid Metals, Bristol, UK, 12 July 1976. [Google Scholar]
- Spitans, S.; Jakovics, A.; Baake, E.; Nacke, B. Numerical modelling of free surface dynamics of conductive melt in the induction crucible furnace. Magnetohydrodynamics 2010, 46, 425–436. [Google Scholar] [CrossRef]
- Spitans, S.; Jakovics, A.; Baake, E.; Nacke, B. Numerical modelling of free surface dynamics of melt in an alternate electromagnetic field. Magnetohydrodynamics 2011, 47, 385–397. [Google Scholar]
- Golak, S.; Przyłucki, R. A simulation of the coupled problem of magnetohydrodynamics and a free surface for liquid metals. Trans. WIT Trans. Eng. Sci. 2009, 56, 67–76. [Google Scholar]
- Blacha, L.; Fornalczyk, A.; Przyłucki, R.; Golak, S. Kinetics of the evaporation process of the volatile component in induction stirred melts. In Proceedings of the 2nd International Conference of Simulation and Modelling of Metallurgical Processes in Steelmaking, Steelsim 2007, Graz, Austria, 12–14 September 2007; Ludwig, A., Ed.; ASMET: Leoben, Austria, 2007; pp. 389–395. [Google Scholar]
- Smalcerz, A.; Przylucki, R. Electromagnetic field analysis of inductor—Robot-workpiece system. Metalurgija. 2013, 52, 223–226. [Google Scholar]
- Wecki, B. Analysis of the Influence of the Contact Area Size between the Liquid Metal Phase and the Gas Phase on the Efficiency of the Metal Refining Process in Induction Crucible Furnaces. Ph.D. Dissertation, Silesian University of Technology, Gliwice, Poland, 2018. [Google Scholar]
- Smalcerz, A.; Blacha, L.; Węcki, B.; Desisa, D.G.; Łabaj, J.; Jodkowski, M. Elimination of zinc from aluminum during remelting in an vacuum induction furnace. Arch. Foundry Eng. 2022, 22, 11–18. [Google Scholar]
- Przyłucki, R.; Golak, S.; Oleksiak, B.; Blacha, L. Influence of an induction furnace’s electric parameters on mass transfer velocity In the liquid phase. Metalurgija 2012, 1, 67–70. [Google Scholar]
- Blacha, L.; Przyłucki, R.; Golak, S.; Oleksiak, B. Influence of the geometry of the arrangement inductor—crucible to the velocity of the transport of mass in the liquid metallic phase mixed inductive. Arch. Civ. Mech. Eng. 2011, 11, 171–179. [Google Scholar]
- Golak, S.; Przyłucki, R.; Barglik, J. Determination of a mass transfer area during metal melting in a vacuum induction furnace. Arch. Metall. Mater. 2014, 59, 287–292. [Google Scholar] [CrossRef]
- Butrymowicz, D.; Manning, J.R.; Read, M.E. Diffusion in copper and copper alloys. J. Phys. Chem. Rev. 1974, 2, 643–653. [Google Scholar]
- Richardson, F.D. Physical Chemistry of Melts in Metallurgy; Academic Press: London, UK, 1974. [Google Scholar]
- HSC Chemistry ver. 6.1. Outocumpu Research Oy. Pori.
- Plewa, J. Przykłady Obliczeń z Teorii Procesów Metalurgicznych; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 1987. [Google Scholar]







| Literature No. | Material | Pb Content, wt.% | Temperature Range, K | Pressure Range, Pa | Pb Removal Rate, % | Mass Transport Coefficient, kPb·105, m·s−1 | |
|---|---|---|---|---|---|---|---|
| 1 | [15] | Cathode copper enriched with Sb and Pb | <0.01 | 1373÷1423 | 13÷26.000 | <90 | - |
| 2 | [16] | Cathode copper | <0.0015 | 1573 | 13 | >90 | - |
| Cathode copper enriched with Sb and Pb | <0.01 | 1503÷1523 | 0.13÷13 | >90 | - | ||
| 3 | [17,18] | Blister copper, anode and cathode copper | 0.027 ÷ 0.33 | 1423÷1523 | 8÷40 | <95 | 1.5÷4.5 |
| 4 | [19,20,21] | Synthetic Cu-Pb alloys, blister copper | <2 | 1373÷1523 | 8–1333 | <90 | 0.6–8.2 |
| 5 | [22] | Synthetic Cu-Pb alloys | <2 | 1473÷1623 | 10.000–55.000 | <48 | 1.09–2.11 |
| Alloy Designation | Alloy Component, wt. %. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Sn | Al | Fe | Mn | Ni | Si | p | Cu | |
| CuPb8 | 8 | 10 | <0.02 | <0.2 | <0.2 | <0.2 | <0.02 | <0.05 | residue |
| CuPb1.9 | 1.9 | 12 | <0.01 | <0.2 | <0.2 | <2 | <0.01 | <0.4 | residue |
| Device Parameters | ICF (VIM) | CCF (ISM 2-200) |
|---|---|---|
| Maximum power | 75 kW | 200 kW |
| Maximum vacuum | 0.01 Pa | 0.001 Pa |
| Vacuum system | Mechanical pump, roots pump, diffusion pump | Mechanical pump, roots pump, diffusion pump |
| Maximum working temperature | 2073 K | 2073 K |
| Crucible volume | 2.5 dm3 | 1 dm3 |
| Alloy with an Initial Lead Content | T, K | P, kW | p, Pa | CkPb wt. % | m, % | N·104, g/cm2·s |
|---|---|---|---|---|---|---|
| Cu-Pb 1.9 wt.% Pb | 1423 | 30 | 1000 | 0.59 | 69.12 | 6.2 |
| 500 | 0.46 | 76.40 | 7.0 | |||
| 100 | 0.34 | 82.55 | 7.8 | |||
| 10 | 0.32 | 83.59 | 7.8 | |||
| 1473 | 40 | 1000 | 0.49 | 77.47 | 6.1 | |
| 500 | 0.46 | 76.20 | 6.4 | |||
| 100 | 0.32 | 83.33 | 7.1 | |||
| 10 | 0.30 | 84.54 | 7.1 | |||
| Cu-Pb 8 wt.% Pb | 1323 | 30 | 1000 | 1.06 | 87.71 | 3.24 |
| 500 | 0.86 | 90.07 | 3.40 | |||
| 100 | 0.53 | 93.91 | 3.54 | |||
| 10 | 0.31 | 96.45 | 3.53 | |||
| 1373 | 40 | 1000 | 0.74 | 91.46 | 3.21 | |
| 500 | 0.55 | 93.70 | 3.23 | |||
| 100 | 0.36 | 95.80 | 3.29 | |||
| 10 | 0.32 | 96.35 | 3.32 |
| Alloy with an Initial Lead Content | T, K | P, kW | p, Pa | CkPb wt. % | m, % | N·104, g/cm2·s |
|---|---|---|---|---|---|---|
| Cu-Pb 1.9 wt.% Pb | 1323 | 170 | 1000 | 0.84 | 55.78 | 2.23 |
| 500 | 0.66 | 65.26 | 2.41 | |||
| 100 | 0.54 | 71.57 | 2.48 | |||
| 10 | 0.39 | 79.47 | 3.17 | |||
| Cu-Pb 8 wt.% Pb | 1273 | 170 | 1000 | 0.99 | 87.62 | 1.61 |
| 500 | 0.77 | 90.37 | 1.63 | |||
| 100 | 0.62 | 92.12 | 1.64 | |||
| 10 | 0.42 | 94.75 | 1.67 |
| Alloy with an Initial Lead Content | T, K | p, Pa | kPb·105, m·s−1 | βlPb·104, m·s−1 | kePb·104, m·s−1 |
|---|---|---|---|---|---|
| Cu-Pb 1.9 wt.% Pb | 1423 | 1000 | 4.66 | 1.76 | 3.33 |
| 500 | 5.15 | 1.76 | 3.33 | ||
| 100 | 5.67 | 1.76 | 3.33 | ||
| 10 | 5.80 | 1.76 | 3.33 | ||
| 1473 | 1000 | 4.47 | 1.78 | 5.11 | |
| 500 | 4.58 | 1.78 | 5.11 | ||
| 100 | 5.16 | 1.78 | 5.11 | ||
| 10 | 5.27 | 1.78 | 5.11 | ||
| Cu-Pb 8 wt.% Pb | 1323 | 1000 | 3.50 | 1.73 | 1.97 |
| 500 | 3.89 | 1.73 | 1.97 | ||
| 100 | 4.73 | 1.73 | 1.97 | ||
| 10 | 5.66 | 1.73 | 1.97 | ||
| 1373 | 1000 | 3.74 | 1.78 | 2.99 | |
| 500 | 4.19 | 1.78 | 2.99 | ||
| 100 | 4.84 | 1.78 | 2.99 | ||
| 10 | 5.05 | 1.78 | 2.99 |
| Alloy with an Initial Lead Content | T, K | p, Pa | kPb·105, m·s−1 | βlPb·104, m·s−1 | kePb·104, m·s−1 |
|---|---|---|---|---|---|
| Cu-Pb 1.9 wt.% Pb | 1323 | 1000 | 1.73 | 1.40 | 1.97 |
| 500 | 1.82 | 1.40 | 1.97 | ||
| 100 | 1.86 | 1.40 | 1.97 | ||
| 10 | 2.12 | 1.40 | 1.97 | ||
| Cu-Pb 8 wt.% Pb | 1273 | 1000 | 1.66 | 1.38 | 1.02 |
| 500 | 1.67 | 1.38 | 1.02 | ||
| 100 | 1.69 | 1.38 | 1.02 | ||
| 10 | 1.94 | 1.38 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blacha, L.; Smalcerz, A.; Wecki, B.; Labaj, J.; Desisa, D.G.; Jodkowski, M. Comparative Analysis of Lead Removal from Liquid Copper by ICF and CCF Refining Technologies. Materials 2022, 15, 7024. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15197024
Blacha L, Smalcerz A, Wecki B, Labaj J, Desisa DG, Jodkowski M. Comparative Analysis of Lead Removal from Liquid Copper by ICF and CCF Refining Technologies. Materials. 2022; 15(19):7024. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15197024
Chicago/Turabian StyleBlacha, Leszek, Albert Smalcerz, Bartosz Wecki, Jerzy Labaj, Debela Geneti Desisa, and Maciej Jodkowski. 2022. "Comparative Analysis of Lead Removal from Liquid Copper by ICF and CCF Refining Technologies" Materials 15, no. 19: 7024. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15197024