Statistical Properties of Protein-Protein Interfaces
Abstract
:1. Introduction
2. Experimental Section
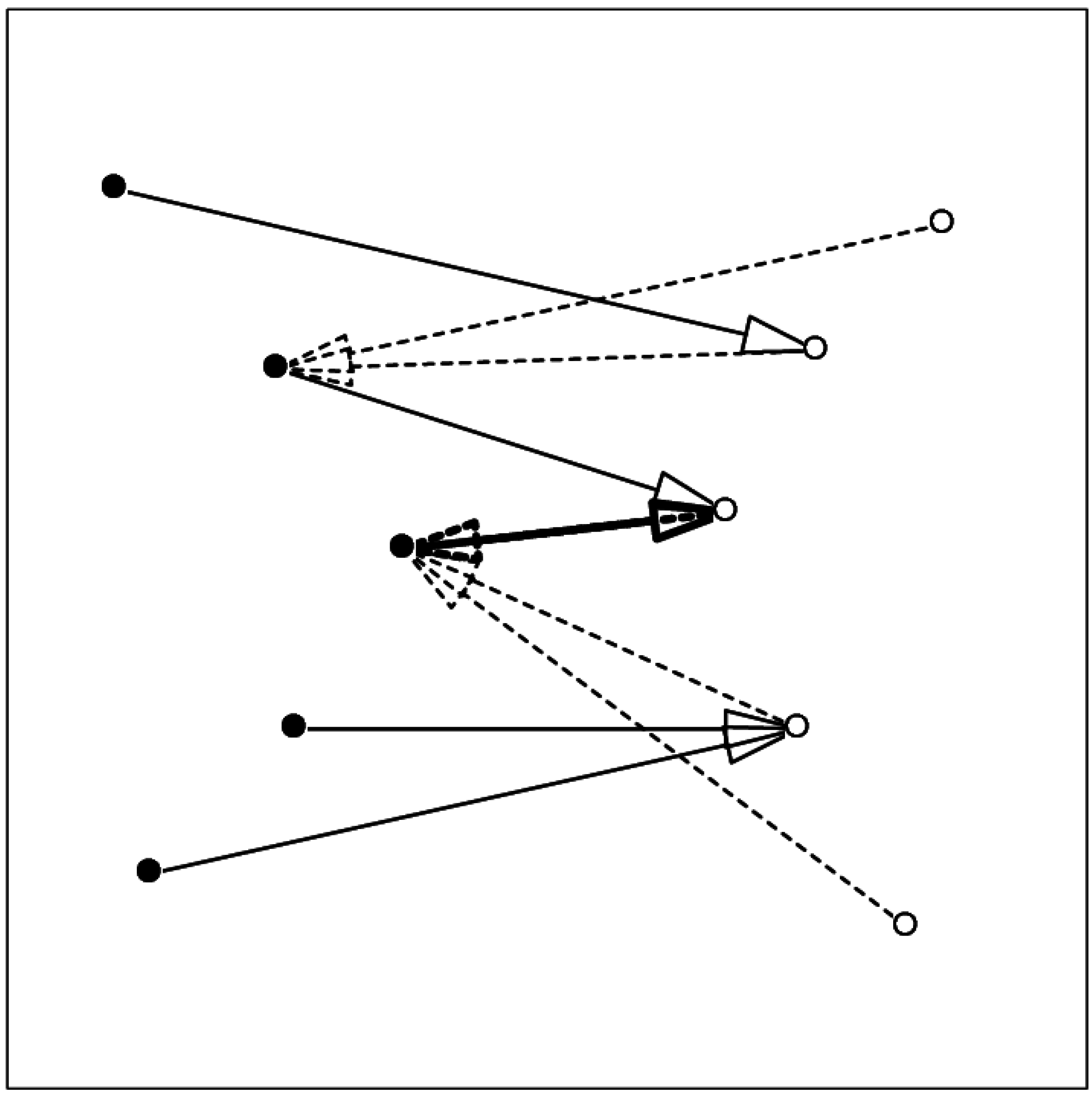
3. Results and Discussion
3.1. Residue Propensities
| %(all) | %(surf) | %(surf)/%(all) | %(cont) | %(cont)/%(all) | %(cont)/%(surf) | |
|---|---|---|---|---|---|---|
| GLY | 6.97 | 6.32 | 0.91 | 5.27 | 0.76 | 0.83 |
| ALA | 6.83 | 5.42 | 0.79 | 4.55 | 0.67 | 0.84 |
| VAL | 6.80 | 5.03 | 0.74 | 5.23 | 0.77 | 1.04 |
| LEU | 8.72 | 6.45 | 0.74 | 6.47 | 0.74 | 1.00 |
| ILE | 5.33 | 3.96 | 0.74 | 4.79 | 0.90 | 1.21 |
| PHE | 3.70 | 2.89 | 0.78 | 4.24 | 1.15 | 1.47 |
| TRP | 2.10 | 2.11 | 1.00 | 2.98 | 1.42 | 1.41 |
| TYR | 4.07 | 4.31 | 1.06 | 6.23 | 1.53 | 1.44 |
| PRO | 5.08 | 5.62 | 1.11 | 5.50 | 1.08 | 0.98 |
| MET | 1.81 | 1.58 | 0.87 | 2.22 | 1.22 | 1.40 |
| SER | 5.88 | 6.19 | 1.05 | 5.17 | 0.88 | 0.83 |
| THR | 6.13 | 6.51 | 1.06 | 5.58 | 0.91 | 0.86 |
| CYS | 1.83 | 1.13 | 0.62 | 1.58 | 0.86 | 1.39 |
| ASN | 4.21 | 4.86 | 1.16 | 5.09 | 1.21 | 1.05 |
| HIS | 2.39 | 2.68 | 1.12 | 3.60 | 1.50 | 1.34 |
| GLN | 4.62 | 5.62 | 1.22 | 6.02 | 1.30 | 1.07 |
| ASP | 5.53 | 6.65 | 1.20 | 6.11 | 1.10 | 0.92 |
| GLU | 6.81 | 8.44 | 1.24 | 6.15 | 0.90 | 0.73 |
| LYS | 6.43 | 8.25 | 1.28 | 5.87 | 0.91 | 0.71 |
| ARG | 4.76 | 5.97 | 1.25 | 7.36 | 1.55 | 1.23 |
| GLY | ALA | VAL | LEU | ILE | PHE | TRP | TYR | PRO | MET | SER | THR | CYS | ASN | HIS | GLN | ASP | GLU | LYS | ARG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLY | 0.65 | |||||||||||||||||||
| ALA | 0.31 | 0.70 | ||||||||||||||||||
| VAL | 0.59 | 0.52 | 1.36 | |||||||||||||||||
| LEU | 0.59 | 0.81 | 1.46 | 1.29 | ||||||||||||||||
| ILE | 0.68 | 0.99 | 1.09 | 2.60 | 2.30 | |||||||||||||||
| PHE | 0.77 | 1.68 | 1.73 | 2.51 | 2.99 | 10.0 | ||||||||||||||
| TRP | 1.19 | 1.99 | 1.78 | 1.73 | 1.23 | 3.24 | 9.12 | |||||||||||||
| TYR | 1.00 | 1.03 | 1.56 | 1.59 | 2.35 | 3.54 | 2.75 | 3.23 | ||||||||||||
| PRO | 1.04 | 0.73 | 1.01 | 1.24 | 0.87 | 1.85 | 2.69 | 2.17 | 0.61 | |||||||||||
| MET | 0.61 | 0.54 | 1.17 | 1.64 | 1.28 | 3.80 | 7.40 | 3.41 | 0.99 | 3.68 | ||||||||||
| SER | 0.46 | 0.45 | 0.89 | 0.45 | 0.39 | 1.36 | 0.95 | 0.87 | 0.79 | 0.93 | 0.58 | |||||||||
| THR | 0.55 | 0.65 | 0.90 | 0.68 | 0.88 | 1.82 | 1.70 | 1.50 | 0.64 | 1.10 | 0.41 | 0.62 | ||||||||
| CYS | 1.55 | 0.92 | 1.09 | 1.60 | 1.25 | 1.31 | 2.25 | 1.05 | 0.45 | 0.75 | 0.35 | 0.34 | 17.2 | |||||||
| ASN | 0.77 | 1.42 | 0.73 | 0.84 | 1.22 | 1.39 | 4.28 | 1.23 | 1.18 | 0.79 | 0.68 | 0.77 | 1.56 | 1.02 | ||||||
| HIS | 2.07 | 0.46 | 1.12 | 0.69 | 1.52 | 1.81 | 2.92 | 3.20 | 0.40 | 1.56 | 0.77 | 0.99 | 2.30 | 1.37 | 2.78 | |||||
| GLN | 0.68 | 0.47 | 1.20 | 0.80 | 1.00 | 1.46 | 4.37 | 1.48 | 1.07 | 1.25 | 0.56 | 1.07 | 0.91 | 0.65 | 1.89 | 1.17 | ||||
| ASP | 0.30 | 0.47 | 0.64 | 0.37 | 0.94 | 0.90 | 2.21 | 1.19 | 0.64 | 0.96 | 0.82 | 0.60 | 0.17 | 0.76 | 2.30 | 0.63 | 0.31 | |||
| GLU | 0.37 | 0.21 | 0.85 | 0.39 | 0.70 | 0.84 | 0.64 | 1.37 | 0.54 | 0.62 | 0.77 | 0.46 | 0.47 | 0.62 | 1.32 | 1.46 | 0.31 | 0.21 | ||
| LYS | 0.38 | 0.32 | 0.45 | 0.41 | 0.71 | 0.96 | 1.00 | 1.37 | 0.26 | 0.79 | 0.50 | 0.53 | 0.92 | 0.71 | 0.52 | 0.49 | 1.12 | 1.16 | 0.47 | |
| ARG | 1.23 | 0.92 | 1.04 | 0.76 | 1.13 | 2.56 | 3.05 | 3.44 | 0.63 | 3.65 | 0.81 | 0.86 | 1.54 | 1.35 | 1.32 | 1.91 | 2.88 | 1.67 | 0.58 | 0.86 |
| GLY | ALA | VAL | LEU | ILE | PHE | TRP | TYR | PRO | MET | SER | THR | CYS | ASN | HIS | GLN | ASP | GLU | LYS | ARG |
3.2. Ruggedness of the Interaction Surface
3.3. Shape of the Interaction Surface
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jones, J.; Thorton, J.M. Analysis of protein-protein interaction sites using surface patches. J. Mol. Biol. 1997, 272, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.S.; Fernandes, P.A.; Ramos, M.J. Hot spots—A review of the protein-protein interface determinant amino-acid residues. Proteins 2007, 68, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Elkayam, T.; Wolfson, H.; Nussinov, R. Protein-protein interactions: Structurally conserved residues distinguish between binding sites and exposed protein surfaces. Proc. Natl. Acad. Sci. USA 2003, 100, 5772–5777. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wu, F.; Jernigan, R.L.; Dobbs, D.; Honavar, V. Characterization of protein-protein interfaces. Protein J. 2008, 27, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yan, C. A comparative analysis of protein interfaces. Protein Pept. Lett. 2010, 17, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Kysilka, J.; Vondrášek, J. Towards a better understanding of the specificity of protein-protein interaction. J. Mol. Recognit. 2012, 25, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.; Zawaira, A.; Saunders, R.; Barrett, C.P.; Noble, M.E. Computational analyses of the surface properties of protein-protein interfaces. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 50–57. [Google Scholar] [CrossRef]
- Reynolds, C.; Damerell, D.; Jones, S. Protorp: A protein-protein interaction analysis server. Bioinformatics 2009, 25, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Vangone, A.; Spinelli, R.; Scarano, V.; Cavallo, L.; Oliva, R. Cocomaps: A web application to analyze and visualize contacts at the interface of biomolecular complexes. Bioinformatics 2011, 27, 2915–2916. [Google Scholar] [CrossRef] [PubMed]
- Guharoy, M.; Chakrabarti, P. Conserved residue clusters at protein-protein interfaces and their use in binding site identification. BMC Bioinform. 2010, 11, 286. [Google Scholar] [CrossRef]
- Eichborn, J.V.; Günther, S.; Preissner, R. Structural features and evolution of protein-protein interactions. Genome Inform. 2010, 22, 1–10. [Google Scholar] [PubMed]
- Duarte, J.M.; Srebniak, A.; Schärer, M.A.; Capitani, G. Protein interface classification by evolutionary analysis. BMC Bioinform. 2912, 13, 334. [Google Scholar] [CrossRef]
- Baskaran, K.; Duarte, J.M.; Biyani, N.; Bliven, S.; Capitani, G. A pdb-wide, evolution-based assessment of protein-protein interfaces. BMC Struct. Biol. 2014. [Google Scholar] [CrossRef]
- Teyra, J.; Doms, A.; Schroeder, M.; Pisabarro, M. Scowlp: A web-based database for detailed characterization and visualization of protein interfaces. BMC Bioinform. 2006, 7, 104. [Google Scholar] [CrossRef]
- Kundrotas, P.; Zhu, Z.; Vakser, I.A. Gwidd: Genome-wide protein docking database. Nucleic Acids Res. 2010, 38, D513–D517. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sawyer, N.; Regan, L. Protein-protein interactions: General trends in the relationship between binding affinity and interfacial buried surface area. Protein Sci. 2013, 22, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Mahdavia, S.; Salehzadeh-Yazdia, A.; Mohadesb, A.; Masoudi-Nejad, A. Computational structure analysis of biomacromolecule complexes by interface geometry. Comput. Biol. Chem. 2013, 47, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Voronoi, G. Nouvelles applications des paramètres continus à la théorie des formes quadratiques. Journal für die reine und angewandte Mathematik 1908, 1908, 97–102. [Google Scholar]
- Bera, I.; Ray, S. A study of interface roughness of heteromeric obligate and non-obligate protein-protein complexes. Bioinformation 2009, 4, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Yura, K.; Hayward, S. The interwinding nature of protein-protein interfaces and its implication for protein complex formation. Bioinformatics 2009, 25, 3108–3113. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Richards, F.M. The interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 1971, 55, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Mardia, K.V.; Jupp, P.E. Directional Statistics; John Wiley & Sons, Ltd: Chichester, UK, 2000. [Google Scholar]
- Mezei, M. A new method for mapping macromolecular topography. J. Mol. Graph. Model. 2003, 21, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Mezei, M.; Zhou, M.M. Dockres: A computer program that analyzes the output of virtual screening of small molecules. Source Code Biol. Med. 2010, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Falchi, F.; Caporuscio, F.; Recanatini, M. Structure-based design of small-molecule protein–protein interaction modulators: The story so far. Future Med. Chem. 2014, 6, 343–357. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezei, M. Statistical Properties of Protein-Protein Interfaces. Algorithms 2015, 8, 92-99. https://0-doi-org.brum.beds.ac.uk/10.3390/a8020092
Mezei M. Statistical Properties of Protein-Protein Interfaces. Algorithms. 2015; 8(2):92-99. https://0-doi-org.brum.beds.ac.uk/10.3390/a8020092
Chicago/Turabian StyleMezei, Mihaly. 2015. "Statistical Properties of Protein-Protein Interfaces" Algorithms 8, no. 2: 92-99. https://0-doi-org.brum.beds.ac.uk/10.3390/a8020092






