Seroprevalence of Hepatitis E Virus in Moose (Alces alces), Reindeer (Rangifer tarandus), Red Deer (Cervus elaphus), Roe Deer (Capreolus capreolus), and Muskoxen (Ovibos moschatus) from Norway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Serological Study
2.3. Statistics
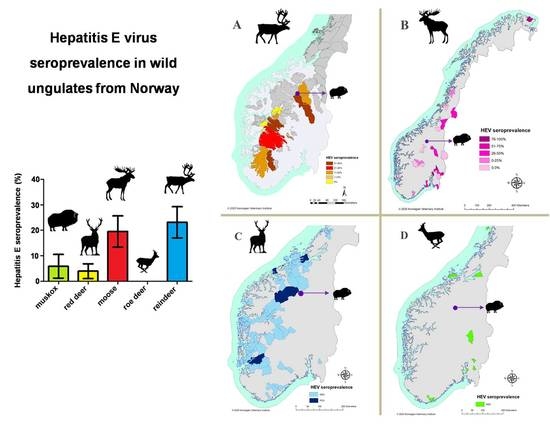
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Said, B.; Ijaz, S.; Chand, M.A.; Kafatos, G.; Tedder, R.; Morgan, D. Hepatitis E virus in England and Wales: Indigenous infection is associated with the consumption of processed pork products. Epidemiol. Infect. 2014, 142, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hepatitis E: Fact Sheet. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 24 September 2020).
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Nishizawa, T.; Tanggis; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the Quasi-Enveloped Hepatitis E Virus Particles Released by the Cellular Exosomal Pathway. J. Virol. 2017, 91, e00822-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapuy-Regaud, S.; Dubois, M.; Plisson-Chastang, C.; Bonnefois, T.; Lhomme, S.; Bertrand-Michel, J.; You, B.; Simoneau, S.; Gleizes, P.E.; Flan, B.; et al. Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie 2017, 141, 70s–79s. [Google Scholar] [CrossRef] [PubMed]
- Purdy, M.A.; Harrison, T.J.; Jameel, S.; Meng, X.J.; Okamoto, H.; Van der Poel, W.; Smith, D.B.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Hepeviridae. J. Gen. Virol. 2017, 98, 2645–2646. [Google Scholar] [CrossRef]
- Saad, M.D.; Hussein, H.A.; Bashandy, M.M.; Kamel, H.H.; Earhart, K.C.; Fryauff, D.J.; Younan, M.; Mohamed, A.H. Hepatitis E virus infection in work horses in Egypt. Infect. Genet. Evol. 2007, 7, 368–373. [Google Scholar] [CrossRef]
- Park, W.J.; Park, B.J.; Ahn, H.S.; Lee, J.B.; Park, S.Y.; Song, C.S.; Lee, S.W.; Yoo, H.S.; Choi, I.S. Hepatitis E virus as an emerging zoonotic pathogen. J. Vet. Sci. 2016, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lanford, R.E.; Walker, C.M.; Lemon, S.M. Nonhuman Primate Models of Hepatitis A Virus and Hepatitis E Virus Infections. Cold Spring Harb. Perspect. Med. 2019, 9, a031815. [Google Scholar] [CrossRef]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.; Morgan, D. Hepatitis E Incident Investigation Team Hepatitis E outbreak on cruise ship. Emerg. Infect. Dis. 2009, 15, 1738–1744. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Govindarajan, S.; Herbert, R.; St Claire, M.; Elkins, W.R.; Cook, A.; Shaver, C.; Beauregard, M.; Swerczek, J.; et al. Pathobiology of hepatitis E: Lessons learned from primate models. Emerg. Microbes Infect. 2013, 2, e9. [Google Scholar] [CrossRef]
- Sato, Y.; Sato, H.; Naka, K.; Furuya, S.; Tsukiji, H.; Kitagawa, K.; Sonoda, Y.; Usui, T.; Sakamoto, H.; Yoshino, S.; et al. A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: Identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch. Virol. 2011, 156, 1345–1358. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizawa, T.; Sato, H.; Sato, Y.; Nagashima, S.; Jirintai; Okamoto, H. Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J. Gen. Virol. 2011, 92 Pt 4, 902–908. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Tsang, A.K.; Joseph, M.; Wong, E.Y.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R.; et al. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Tan, B.H.; Teo, E.C.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic Infection with Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Cao, K.Y.; Wernery, U.; Schountz, T.; Chiu, T.H.; Tsang, A.K.; Wong, P.C.; Wong, E.Y.; et al. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China. Emerg. Infect. Dis. 2016, 22, 2219–2221. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Avellon, A.; Baylis, S.A.; Ciccaglione, A.R.; Couturier, E.; de Sousa, R.; Epštein, J.; Ethelberg, S.; Faber, M.; Fehér, Á.; et al. Hepatitis E virus: Assessment of the epidemiological situation in humans in Europe, 2014/15. J. Clin. Virol. 2016, 82, 9–16. [Google Scholar] [CrossRef]
- Aspinall, E.J.; Couturier, E.; Faber, M.; Said, B.; Ijaz, S.; Tavoschi, L.; Takkinen, J.; Adlhoch, C. The Country Experts Hepatitis E virus infection in Europe: Surveillance and descriptive epidemiology of confirmed cases, 2005 to 2015. Eurosurveillance 2017, 22, 30561. [Google Scholar] [CrossRef] [Green Version]
- Kantala, T.; Maunula, L.; von Bonsdorff, C.H.; Peltomaa, J.; Lappalainen, M. Hepatitis E virus in patients with unexplained hepatitis in Finland. J. Clin. Virol. 2009, 45, 109–113. [Google Scholar] [CrossRef]
- Widén, F.; Sundqvist, L.; Matyi-Toth, A.; Metreveli, G.; Belák, S.; Hallgren, G.; Norder, H. Molecular epidemiology of hepatitis E virus in humans, pigs and wild boars in Sweden. Epidemiol. Infect. 2011, 139, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Baylis, S.A.; Gärtner, T.; Nick, S.; Ovemyr, J.; Blümel, J. Occurrence of hepatitis E virus RNA in plasma donations from Sweden, Germany and the United States. Vox Sang. 2012, 103, 89–90. [Google Scholar] [CrossRef]
- Kantala, T.; Kinnunen, P.M.; Oristo, S.; Jokelainen, P.; Vapalahti, O.; Maunula, L. Hepatitis E Virus Antibodies in Finnish Veterinarians. Zoonoses Public Health 2017, 64, 232–238. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Scientific Opinion on the public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15, 4886. [Google Scholar] [CrossRef]
- Brantsæter, A.B. Hepatitis E—A neglected disease in Norway. Hepatitt E—en neglisjert sykdom I Norge. Tidsskrift for den Norske Laegeforening 2016, 136, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaug, K.; Hagen, I.J.; von der Lippe, B. Three cases of acute hepatitis E virus infection imported into Norway. Scand. J. Infect. Dis. 1994, 26, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Løvdahl, A.; Øverbø, J. En pasient i 20-årene med icterus og smerter i ledd og muskler. Tidsskrift for den Norske Laegeforening 2016, 136, 1651–1652. [Google Scholar] [CrossRef]
- Lange, H.; Øverbø, J.; Borgen, K.; Dudman, S.; Hoddevik, G.; Urdahl, A.M.; Vold, L.; Sjurseth, S.K. Hepatitis E in Norway: Seroprevalence in humans and swine. Epidemiol. Infect. 2017, 145, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Olsøy, I.B.; Henriksen, S.; Weissbach, F.H.; Larsen, M.; Borgen, K.; Abravanel, F.; Kamar, N.; Paulssen, E.J.; Hirsch, H.H.; Rinaldo, C.H. Seroprevalence of hepatitis E virus (HEV) in a general adult population in Northern Norway: The Tromsø study. Med. Microbiol. Immunol. 2019, 208, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Myrmel, M.; Lange, H.; Rimstad, E. A 1-Year Quantitative Survey of Noro-, Adeno-, Human Boca-, and Hepatitis E Viruses in Raw and Secondarily Treated Sewage from Two Plants in Norway. Food Environ. Virol. 2015, 7, 213–223. [Google Scholar] [CrossRef]
- Kreeger, T.J.; Arnemo, J.M. Handbook of Wildlife Chemical Immobilization, 5th ed.; International Wildlife Veterinary Services: Laramie, WY, USA, 2018. [Google Scholar]
- Thiry, D.; Mauroy, A.; Saegerman, C.; Licoppe, A.; Fett, T.; Thomas, I.; Brochier, B.; Thiry, E.; Linden, A. Belgian Wildlife as Potential Zoonotic Reservoir of Hepatitis E Virus. Transbound. Emerg. Dis. 2017, 64, 764–773. [Google Scholar] [CrossRef]
- Development Core Team 3.0.1. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project.org (accessed on 28 September 2020).
- Sarchese, V.; Di Profio, F.; Melegari, I.; Palombieri, A.; Sanchez, S.B.; Arbuatti, A.; Ciuffetelli, M.; Marsilio, F.; Martella, V.; Di Martino, B. Hepatitis E virus in sheep in Italy. Transbound. Emerg. Dis. 2019, 66, 1120–1125. [Google Scholar] [CrossRef]
- Skjerve, E.; Thurfjell, H.; Flø, D.; GrahekOgden, D.; Malmstrø, M.; Nesbakken, T.; Das Neves, C.; Nielsen, A.; Pedersen, H.C.; Robertson, L.; et al.; Wild Boar Population Growth and Expansion—Implications for Biodiversity, Food Safety, and Animal Health in Norway Opinion of the Norwegian Scientific Committee for Food and Environment. 2018. Available online: https://vkm.no/english/riskassessments/allpublications/wildboarpopulationinnorwayimplicationsforhealthandenvironment.4.6c587b9215ef97b46ab50b15.html (accessed on 26 October 2020).
- Statistics Norway. Holdings, Agricultural Area and Livestock. 2020. Available online: https://www.ssb.no/en/statbank/table/05984/ (accessed on 16 November 2020).
- Trogu, T.; Ferrari, N.; Formenti, N.; Filipello, V.; Pedrotti, L.; Viganò, R.; Lanfranchi, P.; Luzzago, C. Low Serologic Prevalences Suggest Sporadic Infections of Hepatitis E Virus in Chamois (Rupicapra rupicapra) and Red Deer (Cervus elaphus) in the Italian Alps. J. Wildl. Dis. 2020, 56, 443–446. [Google Scholar] [CrossRef]
- Minuk, G.Y.; Sun, A.; Sun, D.F.; Uhanova, J.; Nicolle, L.E.; Larke, B.; Giulivi, A. Serological evidence of hepatitis E virus infection in an indigenous North American population. Can. J. Gastroenterol. = J. Can. Gastroenterol. 2007, 21, 439–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weger, S.; Elkin, B.; Lindsay, R.; Bollinger, T.; Crichton, V.; Andonov, A. Hepatitis E Virus Seroprevalence in Free-Ranging Deer in Canada. Transbound. Emerg. Dis. 2017, 64, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Norder, H.; Uhlhorn, H.; Belák, S.; Widén, F. Novel hepatitis E like virus found in Swedish moose. J. Gen. Virol. 2014, 95 Pt 3, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Roth, A.; Lin, J.; Magnius, L.; Karlsson, M.; Belák, S.; Widén, F.; Norder, H. Markers for Ongoing or Previous Hepatitis E Virus Infection Are as Common in Wild Ungulates as in Humans in Sweden. Viruses 2016, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loikkanen, E.; Oristo, S.; Hämäläinen, N.; Jokelainen, P.; Kantala, T.; Sukura, A.; Maunula, L. Antibodies against Hepatitis E virus (HEV) in European moose and white-tailed deer in Finland. Food Environ. Virol. 2020, 12, 333–341. [Google Scholar] [CrossRef]
- Spancerniene, U.; Buitkuviene, J.; Grigas, J.; Pampariene, I.; Salomskas, A.; Cepuliene, R.; Cepuliene, R.; Zymantiene, J.; Stankevicius, A. Seroprevalence of hepatitis E virus in Lithuanian domestic pigs and wildlife. Acta Vet. Brno 2016, 85, 319–327. [Google Scholar] [CrossRef]
- Rutjes, S.A.; Lodder-Verschoor, F.; Lodder, W.J.; van der Giessen, J.; Reesink, H.; Bouwknegt, M.; de Roda Husman, A.M. Seroprevalence and molecular detection of hepatitis E virus in wild boar and red deer in The Netherlands. J. Virol. Methods 2010, 168, 197–206. [Google Scholar] [CrossRef]
- Boadella, M.; Casas, M.; Martín, M.; Vicente, J.; Segalés, J.; de la Fuente, J.; Gortázar, C. Increasing contact with hepatitis E virus in red deer, Spain. Emerg. Infect. Dis. 2010, 16, 1994–1996. [Google Scholar] [CrossRef] [Green Version]
- Kukielka, D.; Rodriguez-Prieto, V.; Vicente, J.; Sánchez-Vizcaíno, J.M. Constant Hepatitis E Virus (HEV) Circulation in Wild Boar and Red Deer in Spain: An Increasing Concern Source of HEV Zoonotic Transmission. Transbound. Emerg. Dis. 2016, 63, e360–e368. [Google Scholar] [CrossRef] [Green Version]
- Di Bartolo, I.; Ponterio, E.; Angeloni, G.; Morandi, F.; Ostanello, F.; Nicoloso, S.; Ruggeri, F.M. Presence of Hepatitis E Virus in a red deer (Cervus elaphus) Population in Central Italy. Transbound. Emerg. Dis. 2017, 64, 137–143. [Google Scholar] [CrossRef]
- Neumann, S.; Hackl, S.S.; Piepenschneider, M.; Vina-Rodriguez, A.; Dremsek, P.; Ulrich, R.G.; Groschup, M.H.; Eiden, M. Serologic and Molecular Survey of Hepatitis E Virus in German Deer Populations. J. Wildl. Dis. 2016, 52, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Palombieri, A.; Robetto, S.; Di Profio, F.; Sarchese, V.; Fruci, P.; Bona, M.C.; Ru, G.; Orusa, R.; Marsilio, F.; Martella, V.; et al. Surveillance Study of Hepatitis E Virus (HEV) in Domestic and Wild Ruminants in Northwestern Italy. Animals 2020, 10, 2351. [Google Scholar] [CrossRef] [PubMed]
- Larska, M.; Krzysiak, M.K.; Jabłoński, A.; Kęsik, J.; Bednarski, M.; Rola, J. Hepatitis E virus antibody prevalence in wildlife in Poland. Zoonoses Public Health 2015, 62, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Anheyer-Behmenburg, H.E.; Szabo, K.; Schotte, U.; Binder, A.; Klein, G.; Johne, R. Hepatitis E Virus in Wild Boars and Spillover Infection in Red and Roe Deer, Germany, 2013–2015. Emerg. Infect. Dis. 2017, 23, 130–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tei, S.; Kitajima, N.; Takahashi, K.; Mishiro, S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003, 362, 371–373. [Google Scholar] [CrossRef]
- Burnie, D.; Wilson, D.E. Animal: The Definitive Visual Guide to the World’s Wildlife; DK Publishing: London, UK, 2005. [Google Scholar]
- Wu, J.; Si, F.; Jiang, C.; Li, T.; Jin, M. Molecular detection of hepatitis E virus in sheep from southern Xinjiang, China. Virus Genes 2015, 50, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Di Martino, B.; Di Profio, F.; Melegari, I.; Sarchese, V.; Robetto, S.; Marsilio, F.; Martella, V. Detection of hepatitis E virus (HEV) in goats. Virus Res. 2016, 225, 69–72. [Google Scholar] [CrossRef]
- Li, T.C.; Chijiwa, K.; Sera, N.; Ishibashi, T.; Etoh, Y.; Shinohara, Y.; Kurata, Y.; Ishida, M.; Sakamoto, S.; Takeda, N.; et al. Hepatitis E virus transmission from wild boar meat. Emerg. Infect. Dis. 2005, 11, 1958–1960. [Google Scholar] [CrossRef]
- Tei, S.; Kitajima, N.; Ohara, S.; Inoue, Y.; Miki, M.; Yamatani, T.; Yamabe, H.; Mishiro, S.; Kinoshita, Y. Consumption of uncooked deer meat as a risk factor for hepatitis E virus infection: An age- and sex-matched case-control study. J. Med. Virol. 2004, 74, 67–70. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, J.M.; Jo, Y.W.; Min, H.J.; Kim, H.J.; Jung, W.T.; Lee, O.J.; Yun, H.; Yoon, Y.S. Genotype-4 hepatitis E in a human after ingesting roe deer meat in South Korea. Clin. Mol. Hepatol. 2013, 19, 309–314. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology 2016, 64, 350–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morellet, N.; Klein, F.; Solberg, E.; Andersen, R. The census and management of populations of ungulates in Europe. In Ungulate Management in Europe: Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 106–143. [Google Scholar]
- Statistics Norway Norwegian Mapping Authority. Mouse Hunting. 2020. Available online: https://www.ssb.no/en/jord-skog-jakt-og-fiskeri/statistikker/elgjakt (accessed on 10 November 2020).
- Statistics Norway Norwegian Mapping Authority. Red deer Hunting. 2020. Available online: https://www.ssb.no/en/jord-skog-jakt-og-fiskeri/statistikker/hjortejakt (accessed on 10 November 2020).
- Statistics Norway Norwegian Mapping Authority. Roe deer Hunting. 2020. Available online: https://www.ssb.no/en/jord-skog-jakt-og-fiskeri/statistikker/srjakt (accessed on 10 November 2020).
- Holand, Ø.; Aikio, P.; Gjøstein, H.; Nieminen, M.; Hove, K.; White, R.G. Modern reindeer dairy farming—the influence of different milking regimes on udder health, milk yield and composition. Small Rumin. Res. 2002, 44, 65–73. [Google Scholar] [CrossRef]
- Nilsson, L.M.; Dahlgren, L.; Johansson, I.; Brustad, M.; Sjölander, P.; Van Guelpen, B. Diet and lifestyle of the Sami of southern Lapland in the 1930s-1950s and today. Int. J. Circumpolar Health 2011, 70, 301–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrenya, N.; Skeie, G.; Melhus, M.; Brustad, M. Food in rural northern Norway in relation to Sami ethnicity: The SAMINOR 2 Clinical Survey. Public Health Nutr. 2018, 21, 2665–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangbru, B.; Seljevoll, K.V. Forvaltningsplan for Moskusbestanden på Dovrefjell. 2017. Available online: https://www.oppdal.kommune.no/globalassets/pdfdokumenter/plan-miljoog-landbruk/miljo/forvaltningsplan-for-moskus-12.12.2017-endelig-versjon.pdf (accessed on 14 October 2020).
- Hartl, J.; Wehmeyer, M.H.; Pischke, S. Acute Hepatitis E: Two Sides of the Same Coin. Viruses 2016, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Cheng, V.; Wong, S.C.; Yip, C.; Wu, S.; Lo, A.; Leung, K.H.; Mak, W.; Cai, J.; Li, X.; et al. Donor-Derived Genotype 4 Hepatitis E Virus Infection, Hong Kong, China, 2018. Emerg. Infect. Dis. 2019, 25, 425–433. [Google Scholar] [CrossRef]
- De Souza, A.J.; Malheiros, A.P.; Soares, M.D.C.; Gomes-Gouvêa, M.S.; Pinho, J.R.; Pereira, W.L.; Sá, L.R. Hallmarks of liver lesions in pigs naturally infected by hepatitis E virus genotype 3. Pesquisa Veterinária Bras. 2018, 38, 65–70. [Google Scholar] [CrossRef]


| Species | County | Nº of Animals | HEV Positive Animals | Age Class | Sex | Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | J | A | U | M | F | U | W | Sp | S | A | NR | ||||
| Reindeer | Agder | 41 | 8 | - | - | 31 | 10 | 5 | 34 | 2 | 30 | 8 | 1 | 2 | - |
| Innlandet | 50 | 12 | - | 4 | 43 | 3 | 4 | 40 | 6 | 41 | 3 | 6 | - | - | |
| Vestfold og Telemark | 9 | 3 | - | - | 9 | - | - | 9 | - | - | 9 | - | - | - | |
| Vestland | 60 | 12 | - | 12 | 41 | 7 | 7 | 53 | - | 36 | 24 | - | - | - | |
| Viken | 6 | 1 | - | 1 | 4 | 1 | - | 6 | - | - | 6 | - | - | - | |
| Not recorded | 20 | 7 | - | 9 | 11 | - | 12 | 8 | - | 9 | 11 | - | - | - | |
| subtotal | 186 | 43 | 0 | 26 | 139 | 21 | 28 | 150 | 8 | 116 | 61 | 7 | 2 | 0 | |
| Moose | Agder | 4 | 1 | - | 2 | 2 | - | 3 | 1 | - | - | - | - | 4 | - |
| Innlandet | 34 | 9 | 7 | 5 | 22 | - | 11 | 21 | 2 | 13 | - | - | 20 | 1 | |
| Nordland | 21 | 1 | 4 | 3 | 9 | 5 | 5 | 16 | - | 16 | - | - | 4 | 1 | |
| Oslo | 1 | 1 | - | - | 1 | - | 1 | - | - | 1 | - | - | - | - | |
| Rogaland | 1 | - | - | 1 | - | - | - | - | 1 | - | - | 1 | - | - | |
| Troms og Finnmark | 11 | 1 | - | - | 11 | - | 1 | 10 | - | 4 | 7 | - | - | - | |
| Trøndelag | 33 | 4 | 12 | 5 | 16 | - | 13 | 17 | 3 | 22 | 0 | 0 | 10 | 1 | |
| Vestfold og Telemark | 12 | 2 | 4 | 3 | 4 | 1 | 9 | 3 | - | - | - | - | 10 | 2 | |
| Viken | 47 | 13 | 5 | 7 | 35 | - | 13 | 33 | 1 | 34 | - | - | 13 | - | |
| subtotal | 164 | 32 | 32 | 26 | 100 | 6 | 56 | 101 | 7 | 90 | 7 | 1 | 61 | 5 | |
| Red deer | Agder | 15 | - | - | - | 3 | 12 | 3 | 12 | - | 15 | - | - | - | - |
| Innlandet | 16 | 2 | 2 | 2 | 12 | - | 11 | 5 | - | - | - | 5 | 11 | - | |
| Møre og Romsdal | 20 | 2 | 1 | 1 | 12 | 6 | 9 | 11 | - | 17 | 3 | - | - | - | |
| Rogaland | 17 | 1 | 6 | - | 10 | 1 | 6 | 11 | - | 10 | 6 | - | 1 | - | |
| Trøndelag | 33 | 2 | 5 | 4 | 20 | 4 | 18 | 15 | - | 15 | 8 | 7 | 3 | - | |
| Vestfold og Telemark | 11 | - | - | - | 5 | 6 | 3 | 8 | - | 11 | - | - | - | - | |
| Vestland | 44 | - | 2 | 1 | 24 | 17 | 12 | 30 | 2 | 25 | 14 | - | 4 | 1 | |
| Viken | 21 | - | - | - | - | 21 | - | 20 | 1 | 20 | - | - | 1 | ||
| subtotal | 177 | 7 | 16 | 8 | 86 | 67 | 62 | 112 | 3 | 113 | 31 | 12 | 19 | 2 | |
| Roe deer | Agder | 9 | - | 1 | 1 | 7 | - | 7 | 2 | - | - | - | 1 | 5 | 3 |
| Innlandet | 10 | - | 2 | 2 | 6 | - | 6 | 4 | - | - | - | - | 10 | - | |
| Nordland | 16 | - | 7 | 1 | 8 | - | 6 | 10 | - | - | - | - | 14 | 2 | |
| Trøndelag | 22 | - | 7 | 2 | 13 | - | 15 | 7 | - | - | - | 7 | 14 | 1 | |
| Vestfold og Telemark | 9 | - | 5 | 1 | 3 | - | 6 | 3 | - | - | - | - | 8 | 1 | |
| Viken | 20 | - | 2 | 6 | 12 | - | 15 | 5 | - | - | - | 4 | 13 | 3 | |
| Subtotal | 86 | 0 | 24 | 13 | 49 | 0 | 55 | 31 | 0 | 0 | 0 | 12 | 64 | 10 | |
| Muskox | Dovrefjell NP subtotal | 102 | 6 | 24 | 13 | 65 | 0 | 56 | 46 | 0 | 12 | 39 | 21 | 1 | 29 |
| Total | 715 | 88 | 96 | 86 | 439 | 94 | 257 | 440 | 18 | 331 | 138 | 53 | 147 | 46 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacristán, C.; Madslien, K.; Sacristán, I.; Klevar, S.; das Neves, C.G. Seroprevalence of Hepatitis E Virus in Moose (Alces alces), Reindeer (Rangifer tarandus), Red Deer (Cervus elaphus), Roe Deer (Capreolus capreolus), and Muskoxen (Ovibos moschatus) from Norway. Viruses 2021, 13, 224. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020224
Sacristán C, Madslien K, Sacristán I, Klevar S, das Neves CG. Seroprevalence of Hepatitis E Virus in Moose (Alces alces), Reindeer (Rangifer tarandus), Red Deer (Cervus elaphus), Roe Deer (Capreolus capreolus), and Muskoxen (Ovibos moschatus) from Norway. Viruses. 2021; 13(2):224. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020224
Chicago/Turabian StyleSacristán, Carlos, Knut Madslien, Irene Sacristán, Siv Klevar, and Carlos G. das Neves. 2021. "Seroprevalence of Hepatitis E Virus in Moose (Alces alces), Reindeer (Rangifer tarandus), Red Deer (Cervus elaphus), Roe Deer (Capreolus capreolus), and Muskoxen (Ovibos moschatus) from Norway" Viruses 13, no. 2: 224. https://0-doi-org.brum.beds.ac.uk/10.3390/v13020224