Amantadine Inhibits SARS-CoV-2 In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Compounds
2.2. S-Protein—ACE2 Binding Assay
2.3. Cell Line
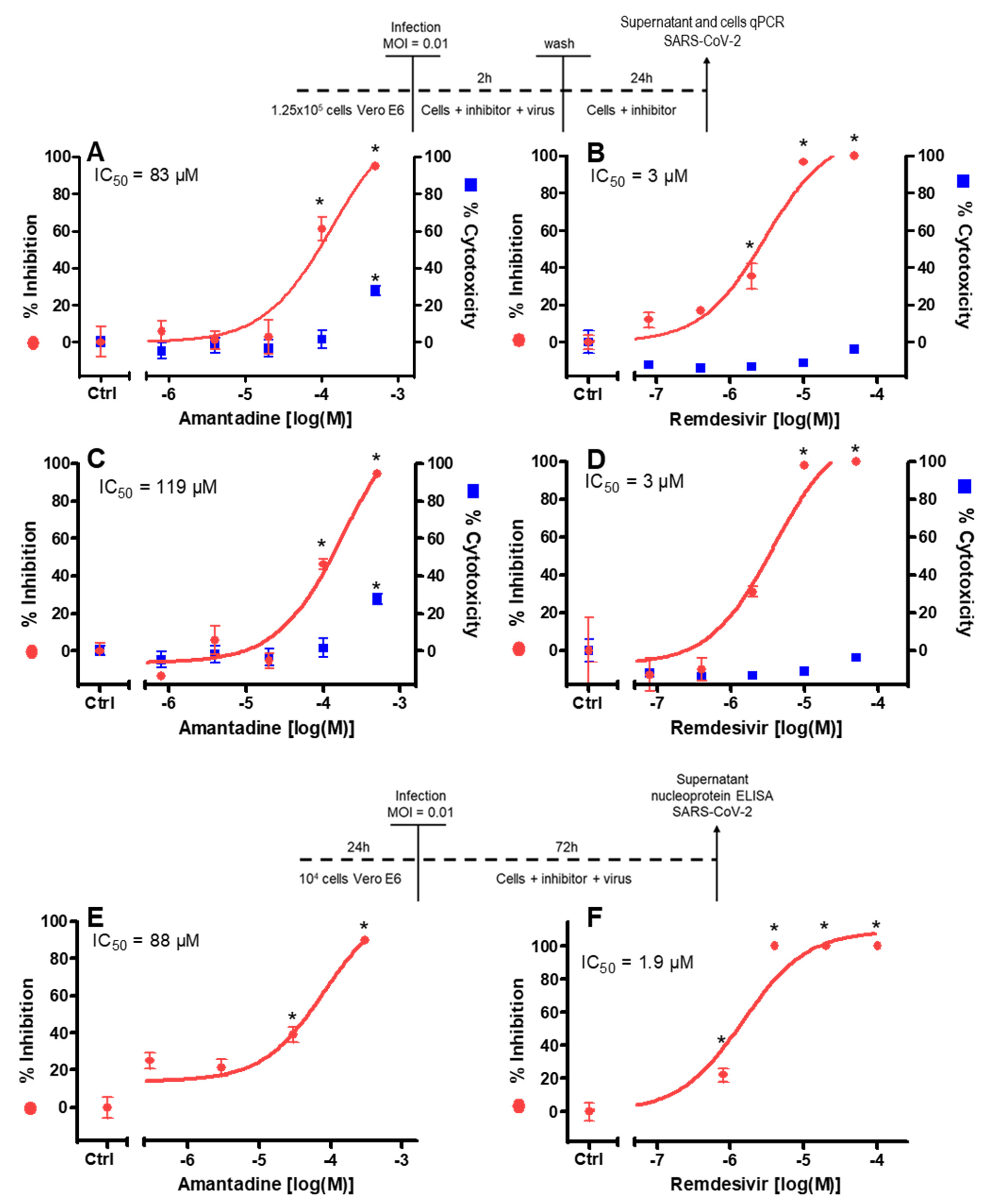
2.4. Antiviral Activity Assay with RT-PCR Readout (1st Experiment)
2.5. Antiviral Activity Assay with Nucleocapsid Protein Readout (2nd Experiment)
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [Green Version]
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Perry, C.; Pan, C.; Hosain, R.; Mahmood, A.; Davis, J.D.; Turner, K.C.; Hooper, A.T.; Hamilton, J.D.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2020, 384, 238–251. [Google Scholar] [CrossRef]
- Cohen, M.S. Monoclonal Antibodies to Disrupt Progression of Early Covid-19 Infection. New Engl. J. Med. 2021, 384, 289–291. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, M.; Yin, L.; Wang, K.; Zhou, Y.; Zhou, M.; Lu, Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 2020, 56, 106080. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernández García, C.; Kieny, M.P.; Malekzadeh, R.; et al. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. New Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Danysz, W.; Dekundy, A.; Scheschonka, A.; Riederer, P. Amantadine: Reappraisal of a timeless diamond—target updates and novel therapeutic potentials. J. Neural. Transm. 2021, 128, 127–169. [Google Scholar] [CrossRef]
- Hay, A.; Wolstenholme, A.; Skehel, J.; Smith, M. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985, 4, 3021–3024. [Google Scholar] [CrossRef]
- Fleming, D.M. Managing influenza: Amantadine, rimantadine and beyond. Int. J. Clin. Pr. 2001, 55, 189–195. [Google Scholar]
- Parsons, C.; Quack, G.; Bresink, I.; Baran, L.; Przegalinski, E.; Kostowski, W.; Krzascik, P.; Hartmann, S.; Danysz, W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 1995, 34, 1239–1258. [Google Scholar] [CrossRef]
- Cady, S.D.; Luo, W.; Hu, F.; Hong, M. Structure and Function of the Influenza A M2 Proton Channel. Biochemistry 2009, 48, 7356–7364. [Google Scholar] [CrossRef] [Green Version]
- Pielak, R.M.; Chou, J.J. Flu channel drug resistance: A tale of two sites. Protein Cell 2010, 1, 246–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeppesen, M.G.; Toft-Bertelsen, T.L.; Kledal, T.N.; Rosenkilde, M.M. Amantadine has potential for the treatment of COVID-19 because its targets known and novel ion channels encoded by SARS-CoV-2. Res. Sq. 2021, 1–8, preprint. [Google Scholar] [CrossRef]
- Torres, J.; Maheswari, U.; Parthasarathy, K.; Ng, L.; Liu, D.X.; Gong, X. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 2007, 16, 2065–2071. [Google Scholar] [CrossRef]
- Wilson, L.; Mckinlay, C.; Gage, P.; Ewart, G. SARS coronavirus E protein forms cation-selective ion channels. Virology 2004, 330, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Brison, E.; Jacomy, H.; Desforges, M.; Talbot, P.J. Novel Treatment with Neuroprotective and Antiviral Properties against a Neuroinvasive Human Respiratory Virus. J. Virol. 2013, 88, 1548–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandala, V.S.; McKay, M.J.; Shcherbakov, A.A.; Dregni, A.J.; Kolocouris, A.; Hong, M. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 2020, 27, 1202–1208. [Google Scholar] [CrossRef]
- Lu, H.; Yan, P.; Xiong, W.; Wang, J.; Liu, X. Genomic characterization of a novel virulent phage infecting Shigella fiexneri and isolated from sewage. Virus Res. 2020, 283, 197983. [Google Scholar] [CrossRef]
- Surya, W.; Li, Y.; Verdià-Bàguena, C.; Aguilella, V.M.; Torres, J. MERS coronavirus envelope protein has a single transmembrane domain that forms pentameric ion channels. Virus Res. 2015, 201, 61–66. [Google Scholar] [CrossRef]
- Tomar, P.P.S.; Arkin, I.T. SARS-CoV-2 E protein is a potential ion channel that can be inhibited by Gliclazide and Memantine. Biochem. Biophys. Res. Commun. 2020, 530, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Lalchhandama, K. The chronicles of coronaviruses: The electron microscope, the doughnut, and the spike. Sci. Vis. 2020, 20, 78–92. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Verdiá-Báguena, C.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Castaño-Rodriguez, C.; Fernandez-Delgado, R.; Torres, J.; Aguilella, V.M.; Enjuanes, L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 2015, 485, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. 2008–2009 Influenza Season Week 35 ending September 5, 2009 Report; CDC FluView: A Weekly Influenza Surveillance Report Prepared by the Influenza Division; Center for Disease Control: Atlanta, GA, USA, 2009. Available online: https://stacks.cdc.gov/view/cdc/80040 (accessed on 15 February 2021).
- Cho, A.; Saunders, O.L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J.E.; Feng, J.Y.; Ray, A.S.; Kim, C.U. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorganic Med. Chem. Lett. 2012, 22, 2705–2707. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, N.; Moss, W.J. Encouraging results from phase 1/2 COVID-19 vaccine trials. Lancet 2020, 396, 448–449. [Google Scholar] [CrossRef]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.W.; Van Der Meer, Y.; Caly, L.; Druce, J.; De Vries, J.J.C.; Kikkert, M.; Bárcena, M.; et al. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef]
- Pruijssers, A.J.; George, A.S.; Schäfer, A.; Leist, S.R.; Gralinksi, L.E.; Dinnon, K.H.; Yount, B.L.; Agostini, M.L.; Stevens, L.J.; Chappell, J.D.; et al. Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice. Cell Rep. 2020, 32, 107940. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, M.; Haass, A.; Jacobi, P.; Schimrigk, K. Amantadine sulphate in treating Parkinson’s disease: Clinical effects, psychometric tests and serum concentrations. J. Neurol. 1989, 236, 153–156. [Google Scholar] [CrossRef]
- Hayden, F.G.; Hall, W.J.; Douglas, R.G.; Speers, D.M. Amantadine aerosols in normal volunteers: Pharmacology and safety testing. Antimicrob. Agents Chemother. 1979, 16, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Albrektson, K.; Mandviwala, A.; Donatelli, C.; Soliman, D.; Lackamp, J.; Teba, C. Amantadine Toxicity: Uncovering a Rare Cause of Severe Encephalopathy. D46. Crit. Care Case Rep. Toxicol. Poisonings 2018, 197, A6912. [Google Scholar]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef]
- Higgins, T.S.; Wu, A.W.; Illing, E.A.; Sokoloski, K.J.; Weaver, B.A.; Anthony, B.P.; Hughes, N.; Ting, J.Y. Intranasal Antiviral Drug Delivery and Coronavirus Disease 2019 (COVID-19): A State of the Art Review. Otolaryngol. Neck Surg. 2020, 163, 682–694. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Khaleeq, A.; Syeda, H. Docking Prediction of Amantadine in the Receptor Binding Domain of Spike Protein of SARS-CoV-2. ACS Pharmacol. Transl. Sci. 2020, 3, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.S.; Choudhury, P.P.; Roy, B.; Jana, S.S. Missense mutations in SARS-CoV2 genomes from Indian patients. Genomics 2020, 112, 4622–4627. [Google Scholar] [CrossRef]
- Rambaut, A.; Loman, N.; Pybus, O.; Barclay, W.; Barrett, J.; Carabelli, A.; Connor, T.; Peacock, T.; Robertson, D.L.; Volz, E.; et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. ARTIC Netw. 2021, 1–9, preprint. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fink, K.; Nitsche, A.; Neumann, M.; Grossegesse, M.; Eisele, K.-H.; Danysz, W. Amantadine Inhibits SARS-CoV-2 In Vitro. Viruses 2021, 13, 539. https://0-doi-org.brum.beds.ac.uk/10.3390/v13040539
Fink K, Nitsche A, Neumann M, Grossegesse M, Eisele K-H, Danysz W. Amantadine Inhibits SARS-CoV-2 In Vitro. Viruses. 2021; 13(4):539. https://0-doi-org.brum.beds.ac.uk/10.3390/v13040539
Chicago/Turabian StyleFink, Klaus, Andreas Nitsche, Markus Neumann, Marica Grossegesse, Karl-Heinz Eisele, and Wojciech Danysz. 2021. "Amantadine Inhibits SARS-CoV-2 In Vitro" Viruses 13, no. 4: 539. https://0-doi-org.brum.beds.ac.uk/10.3390/v13040539





