Impact of COVID-19 Mitigation Measures on Mosquito-Borne Diseases in 2020 in Queensland, Australia
Abstract
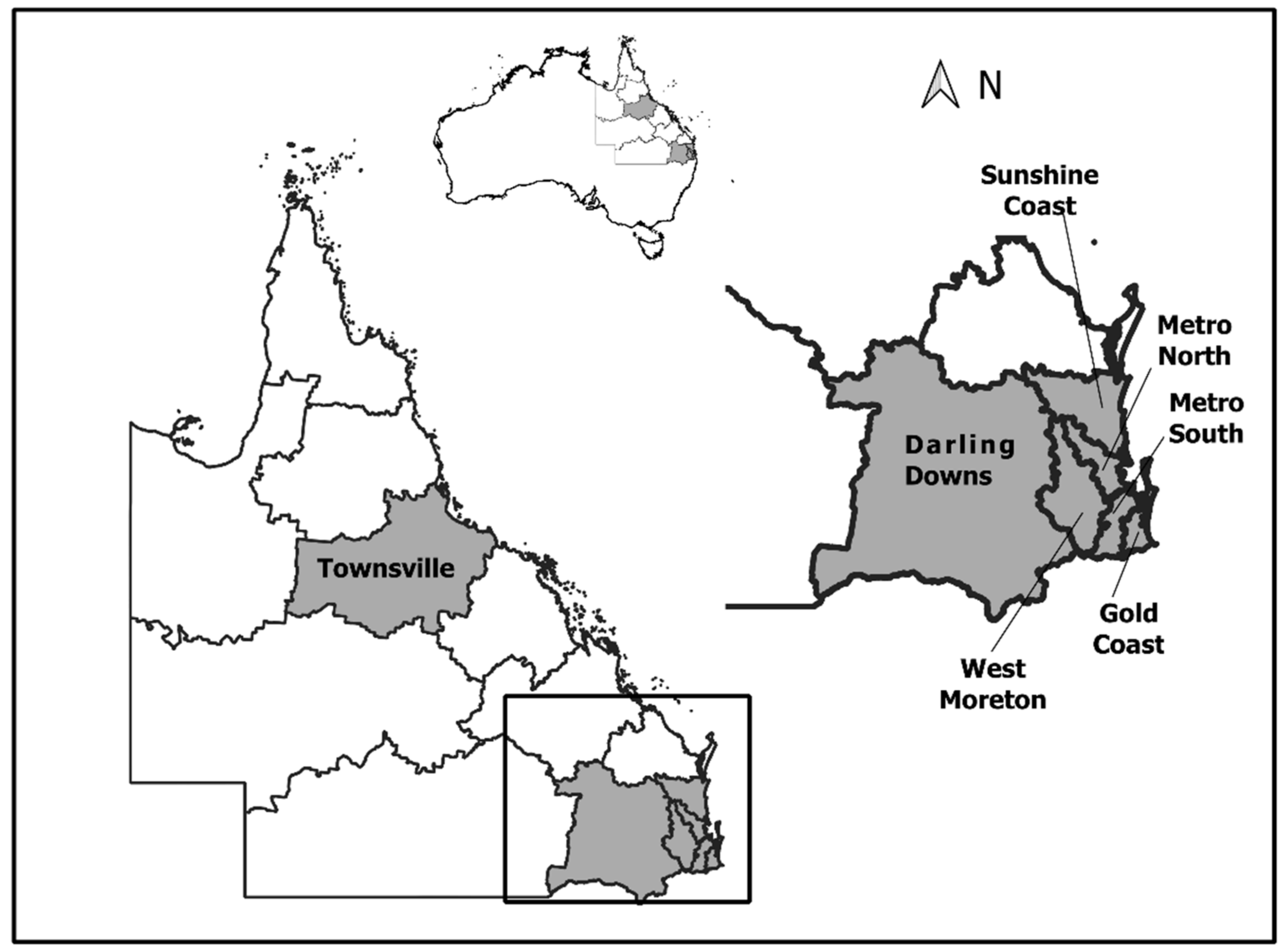
:1. Introduction
2. Unseasonal RRV Transmission
3. Importation of Exotic Pathogens by Overseas Travellers
4. Exotic and Invasive Mosquitoes
5. Impact on Workforce
6. Communicating Evidence That Mosquitoes Are Unlikely to Be Vectors of SARS-CoV-2
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachs, J.D.; Karim, S.A.; Aknin, L.; Allen, J.; Brosbøl, K.; Cuevas Barron, G.; Daszak, P.; María Fernanda Espinosa, M.F.; Gaspar, V.; Gaviria, A.; et al. Priorities for the COVID-19 pandemic at the start of 2021: Statement of the Lancet COVID-19 Commission. Lancet 2021, 397, 947–950. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.; Roberts, J.; Bond, K.; Tran, T.; Kostecki, R.; Yoga, Y.; Naughton, W.; Taiaroa, G.; Seemann, T.; et al. Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia. Med. J. Aust. 2020, 212, 459–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Government Department of Health. Coronavirus (COVID-19) Current Situation and Case Numbers. Available online: https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert/coronavirus-covid-19-current-situation-and-case-numbers (accessed on 5 June 2021).
- Price, D.J.; Shearer, F.M.; Meehan, M.T.; McBryde, E.; Moss, R.; Golding, N.; Conway, E.J.; Dawson, P.; Cromer, D.; Wood, J.; et al. Early analysis of the Australian COVID-19 epidemic. Elife 2020, 9, e58785. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Arboviruses and COVID-19: The need for a holistic view. Lancet Microbe 2020, 1, e136. [Google Scholar] [CrossRef]
- Hogan, A.B.; Jewell, B.L.; Sherrard-Smith, E.; Vesga, J.F.; Watson, O.J.; Whittaker, C.; Hamlet, A.; Smith, J.A.; Winskill, P.; Verity, R.; et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: A modelling study. Lancet Glob. Health 2020, 8, e1132–e1141. [Google Scholar] [CrossRef]
- Weiss, D.J.; Bertozzi-Villa, A.; Rumisha, S.F.; Amratia, P.; Arambepola, R.; Battle, K.E.; Cameron, E.; Chestnutt, E.; Gibson, H.S.; Harris, J.; et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: A geospatial modelling analysis. Lancet Infect. Dis. 2021, 21, 59–69. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Tissera, H.; Ooi, E.E.; Coloma, J.; Scott, T.W.; Gubler, D.J. Preventing dengue epidemics during the COVID-19 pandemic. Am. J. Trop. Med. Hyg. 2020, 103, 570–571. [Google Scholar] [CrossRef]
- Van den Hurk, A.F.; Craig, S.B.; Tulsiani, S.M.; Jansen, C.C. Emerging tropical diseases in Australia. Part 4. Mosquitoborne diseases. Ann. Trop. Med. Parasitol. 2010, 104, 623–640. [Google Scholar] [CrossRef]
- Australian Government Department of Health. National Notifiable Diseases Surveillance System. Available online: http://www9.health.gov.au/cda/source/cda-index.cfm (accessed on 5 June 2021).
- Jansen, C.C.; Shivas, M.A.; May, F.J.; Pyke, A.T.; Onn, M.B.; Lodo, K.; Hall-Mendelin, S.; McMahon, J.L.; Montgomery, B.L.; Darbro, J.M.; et al. Epidemiologic, entomologic, and virologic factors of the 2014-15 Ross River virus outbreak, Queensland, Australia. Emerg. Infect. Dis. 2019, 25, 2243–2252. [Google Scholar] [CrossRef] [Green Version]
- Flexman, J.P.; Smith, D.W.; Mackenzie, J.S.; Fraser, J.R.; Bass, S.P.; Hueston, L.; Lindsay, M.D.A.; Cunningham, A.L. A comparison of the diseases caused by Ross River virus and Barmah Forest virus. Med. J. Aust. 1998, 169, 159–163. [Google Scholar] [CrossRef]
- Fraser, J.R. Epidemic polyarthritis and Ross River virus disease. Clin. Rheum. Dis. 1986, 12, 369–388. [Google Scholar] [CrossRef]
- Claflin, S.B.; Webb, C.E. Ross River virus: Many vectors and unusual hosts make for an unpredictable pathogen. PLoS Pathog. 2015, 11, e1005070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly-Hope, L.A.; Purdie, D.M.; Kay, B.H. Ross River virus disease in Australia, 1886-1998, with analysis of risk factors associated with outbreaks. J. Med. Entomol. 2004, 41, 133–150. [Google Scholar] [CrossRef]
- Murphy, A.K.; Clennon, J.A.; Vazquez-Prokopec, G.; Jansen, C.C.; Frentiu, F.D.; Hafner, L.M.; Hu, W.; Devine, G.J. Spatial and temporal patterns of Ross River virus in south east Queensland, Australia: Identification of hot spots at the rural-urban interface. BMC Infect. Dis. 2020, 20, 722. [Google Scholar] [CrossRef]
- Australian Government Bureau of Meteorology. Climate Data Online. Available online: http://www.bom.gov.au/climate/data/index.shtml (accessed on 5 June 2021).
- Queensland Health. Chief Health Officer Public Health Directions. Available online: https://www.health.qld.gov.au/system-governance/legislation/cho-public-health-directions-under-expanded-public-health-act-powers (accessed on 5 June 2021).
- Queensland Health. Notifiable Conditions Register. Available online: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/notifiable-conditions/register (accessed on 5 June 2021).
- Harley, D.; Ritchie, S.; Bain, C.; Sleigh, A.C. Risks for Ross River virus disease in tropical Australia. Int J. Epidemiol. 2005, 34, 548–555. [Google Scholar] [CrossRef]
- Gemba. Physical Activity during the COVID-19 Lockdown [Internet]. Gemba; 2020 [cited 11 June 2020]. Available online: http://thegembagroup.com/wp-content/uploads/2020/04/GEMBA_COVID-19_Insights_Sports-and-Physical-Activity-Participation__290420.pdf (accessed on 5 June 2021).
- Webb, C.E. Reflections on a highly unusual summer: Bushfires, COVID-19 and mosquito-borne disease in NSW, Australia. Public Health Res. Pract. 2020, 30, 3042027. [Google Scholar] [CrossRef]
- ProMed-Mail. Tick-Borne Encephalitis—Germany (03): Baden-Wurttemberg, Bavaria. Available online: https://promedmail.org/ (accessed on 5 June 2021).
- Hanna, J.N.; Ritchie, S.A.; Eisen, D.P.; Cooper, R.D.; Brookes, D.L.; Montgomery, B.L. An outbreak of Plasmodium vivax malaria in Far North Queensland, 2002. Med. J. Aust. 2004, 180, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Pyke, A.T.; Hall-Mendelin, S.; Day, A.; Mores, C.N.; Christofferson, R.C.; Gubler, D.J.; Bennett, S.N.; van den Hurk, A.F. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS ONE 2013, 8, e68137. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Overseas Arrivals and Departures, Australia. Available online: https://www.abs.gov.au/statistics/industry/tourism-and-transport/overseas-arrivals-and-departures-australia/latest-release (accessed on 5 June 2021).
- Beebe, N.W.; Cooper, R.D.; Mottram, P.; Sweeney, A.W. Australia’s dengue risk driven by human adaptation to climate change. PLoS Negl. Trop. Dis. 2009, 3, e429. [Google Scholar] [CrossRef]
- Van den Hurk, A.F.; Nicholson, J.; Beebe, N.W.; Davis, J.; Muzari, O.M.; Russell, R.C.; Devine, G.J.; Ritchie, S.A. Ten years of the Tiger: Aedes albopictus presence in Australia since its discovery in the Torres Strait in 2005. One Health 2016, 2, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, B.L.; Shivas, M.A.; Hall-Mendelin, S.; Edwards, J.; Hamilton, N.A.; Jansen, C.C.; McMahon, J.L.; Warrilow, D.; van den Hurk, A.F. Rapid Surveillance for Vector Presence (RSVP): Development of a novel system for detecting Aedes aegypti and Aedes albopictus. PLoS Negl. Trop. Dis. 2017, 11, e0005505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sly, A.; Mack, C. Protecting Australia from disease vectors: Exotic mosquito management at the border. Microbiol. Aust. 2018, 39, 108–110. [Google Scholar] [CrossRef]
- Schmidt, T.L.; Chung, J.; van Rooyen, A.R.; Sly, A.; Weeks, A.R.; Hoffmann, A.A. Incursion pathways of the Asian tiger mosquito (Aedes albopictus) into Australia contrast sharply with those of the yellow fever mosquito (Aedes aegypti). Pest Manag. Sci. 2020, 76, 4202–4209. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Moore, P.; Carruthers, M.; Williams, C.; Montgomery, B.; Foley, P.; Ahboo, S.; van den Hurk, A.F.; Lindsay, M.D.; Cooper, R.D.; et al. Discovery of a widespread infestation of Aedes albopictus in the Torres Strait, Australia. J. Am. Mosq. Control Assoc. 2006, 22, 358–365. [Google Scholar] [CrossRef]
- Muzari, M.O.; Devine, G.; Davis, J.; Crunkhorn, B.; van den Hurk, A.; Whelan, P.; Russell, R.; Walker, J.; Horne, P.; Ehlers, G.; et al. Holding back the tiger: Successful control program protects Australia from Aedes albopictus expansion. PLoS Negl. Trop. Dis. 2017, 11, e0005286. [Google Scholar] [CrossRef]
- Moise, I.K.; Ortiz-Whittingham, L.R.; Omachonu, V.; Clark, M.; Xue, R.D. Fighting mosquito bite during a crisis: Capabilities of Florida mosquito control districts during the COVID-19 pandemic. BMC Public Health 2021, 21, 687. [Google Scholar] [CrossRef]
- Fortuna, C.; Montarsi, F.; Severini, F.; Marsili, G.; Toma, L.; Amendola, A.; Bertola, M.; Michelutti, A.; Ravagnan, S.; Capelli, G.; et al. The common European mosquitoes Culex pipiens and Aedes albopictus are unable to transmit SARS-CoV-2 after a natural-mimicking challenge with infected blood. Parasites Vectors 2021, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Vanlandingham, D.L.; Bilyeu, A.N.; Sharp, H.M.; Hettenbach, S.M.; Higgs, S. SARS-CoV-2 failure to infect or replicate in mosquitoes: An extreme challenge. Sci. Rep. 2020, 10, 11915. [Google Scholar] [CrossRef] [PubMed]
- Queensland Health. Public Health Act 2005; Queensland Health: Brisbane, QLD, Australia, 2021; p. 395.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, C.C.; Darbro, J.M.; Birrell, F.A.; Shivas, M.A.; van den Hurk, A.F. Impact of COVID-19 Mitigation Measures on Mosquito-Borne Diseases in 2020 in Queensland, Australia. Viruses 2021, 13, 1150. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061150
Jansen CC, Darbro JM, Birrell FA, Shivas MA, van den Hurk AF. Impact of COVID-19 Mitigation Measures on Mosquito-Borne Diseases in 2020 in Queensland, Australia. Viruses. 2021; 13(6):1150. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061150
Chicago/Turabian StyleJansen, Cassie C., Jonathan M. Darbro, Frances A. Birrell, Martin A. Shivas, and Andrew F. van den Hurk. 2021. "Impact of COVID-19 Mitigation Measures on Mosquito-Borne Diseases in 2020 in Queensland, Australia" Viruses 13, no. 6: 1150. https://0-doi-org.brum.beds.ac.uk/10.3390/v13061150





