Comparative Assessment of In Vitro and In Silico Methods for Aerodynamic Characterization of Powders for Inhalation
Abstract
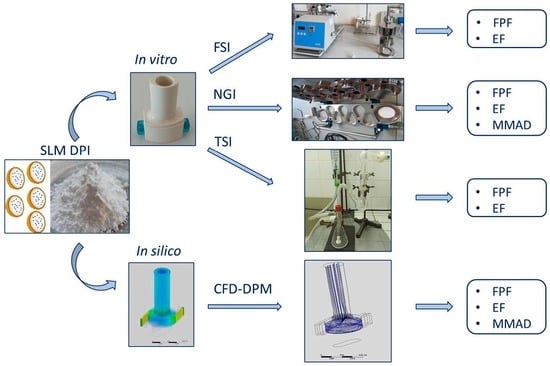
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. SLM DPIs Micromeritic Properties
2.2.2. In Vitro Aerodynamic Assessment of SLM DPIs
Fast Screening Impactor
Next Generation Impactor
Twin Stage Impinger
2.2.3. CFD-DPM Modeling
2.2.4. Aerodynamic Particle Size Distribution of CFD-DPM Generated Aerosol Data
2.2.5. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a | Distance along the contact surface from the particle center |
| a1 | Empirically determined constant |
| ACI | Andersen cascade impactor |
| b | Deformation of the particle normal to the surface |
| CFD | Computational fluid dynamics |
| Cu | Cunningham correction factor |
| dae | Aerodynamic diameter |
| dg | Geometric diameter |
| dp | Particle diameter |
| DPIs | Dry powders for inhalation and dry powder inhalers |
| DEM | Discrete element method |
| DPM | Discrete phase modeling and discrete phase model |
| E | El Batch parameter |
| ED | Emitted dose |
| EF | Emitted fraction |
| Es | Young’s modulus for the inhaler wall surface |
| Ep | Young’s modulus for particle |
| f | Correction factor for the near wall |
| FD | Drag force |
| FL | Lift force |
| Fst | Adhesion force |
| FPD | Fine particle dose |
| FPF | Fine particle fraction |
| FSI | Fast screening impactor |
| GSD | Geometric standard deviation |
| HPLC | High performance liquid chromatography |
| HPMC | Hydroxypropylmethylcellulose |
| IP | Induction port |
| k | Turbulent kinetic energy |
| ks | Static coefficient of friction |
| LES | Large eddy simulations |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MD | Metered dose |
| MDIs | Metered dose inhalers |
| MMAD | Mass median aerodynamic diameter |
| MOC | Micro-orifice collector |
| MSLI | Multistage liquid impinger |
| N | Number of reference/test values |
| NGI | Next generation impactor |
| NRMSE | Normalized root mean square errror |
| Mean of the observed (reference) values | |
| Oi | Observed or reference values |
| Pi | Predicted or test values |
| R2 | Correlation coefficient |
| RANS | Reynolds Averaged Navier Stokes |
| RMSE | Root mean square error |
| SLMs | Solid lipid microparticles |
| SS | Salbutamol-sulphate |
| SST | Shear stress transport |
| TSI | Twin stage impinger |
| UDFs | User defined functions |
| Reynolds stress tensor | |
| vcr | Capture velocity |
| vn | Normal impact velocity |
| vp | Poisson’s ratio for particle |
| vs | Poisson’s ratio for the inhaler wall surface |
| vt | Turbulent kinematic viscosity |
| WA | Work of adhesion |
| µ | Dynamic viscosity of fluid |
| ρ | Air density |
| ρp | Particle density |
| ω | Specific turbulence dissipation rate |
References
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.C.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.L.; Carroll, W.; Izquierdo Alonso, J.L.; Keller, C.; Lavorini, F.; Lehtimäki, L. Understanding dry powder inhalers: Key technical and patient preference attributes. Adv. Ther. 2019, 36, 2547–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical stability of dry powder inhaler formulations. Expert Opin. Drug Deliv. 2020, 17, 77–96. [Google Scholar] [CrossRef] [Green Version]
- Nurbaeti, S.N.; Brillault, J.; Tewes, F.; Olivier, J.C. Sustained-release microparticle dry powders of chloramphenicol palmitate or thiamphenicol palmitate prodrugs for lung delivery as aerosols. Eur. J. Pharm. Sci. 2019, 138, 105028. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.; Patil, P.; Rajput, R.; Mujumdar, A.; Naik, J. Preparation and characterization of sustained release pirfenidone loaded microparticles for pulmonary drug delivery: Spray drying approach. Dry. Technol. 2020, 39, 337–347. [Google Scholar] [CrossRef]
- Chogale, M.M.; Dhoble, S.B.; Patravale, V.B. A triple combination ‘nano’ dry powder inhaler for tuberculosis: In vitro and in vivo pulmonary characterization. Drug Deliv. Transl. Res. 2021, 11, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Mezzena, M.; Scalia, S.; Young, P.M.; Traini, D. Solid lipid budesonide microparticles for controlled release inhalation therapy. AAPS J. 2009, 11, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Ben-Jebria, A.; Chen, D.; Eskew, M.L.; Vanbever, R.; Langer, R.; Edwards, D.A. Large porous particles for sustained protection from carbachol-induced bronchoconstriction in guinea pigs. Pharm. Res. 1999, 16, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Vanbever, R.; Ben-Jebria, A.; Mintzes, J.D.; Langer, R.; Edwards, D.A. Sustained release of insulin from insoluble inhaled particles. Drug Dev. Res. 1999, 48, 178–185. [Google Scholar] [CrossRef]
- Newhouse, M.T.; Hirst, P.H.; Duddu, S.P.; Walter, Y.H.; Tarara, T.E.; Clark, A.R.; Weers, J.G. Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest 2003, 124, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Steckel, H.; Brandes, H.G. A novel spray-drying technique to produce low density particles for pulmonary delivery. Int. J. Pharm. 2004, 278, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.O.; Pannu, R.K.; Kellaway, I.W. Novel sustained release microspheres for pulmonary drug delivery. J. Control. Release 2005, 104, 79–90. [Google Scholar] [CrossRef]
- Sebti, T.; Amighi, K. Preparation and in vitro evaluation of lipidic carriers and fillers for inhalation. Eur. J. Pharm. Biopharm. 2006, 63, 51–58. [Google Scholar] [CrossRef]
- Jaspart, S.; Bertholet, P.; Piel, G.; Dogné, J.M.; Delattre, L.; Evrard, B. Solid lipid microparticles as a sustained release system for pulmonary drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 47–56. [Google Scholar] [CrossRef]
- Depreter, F.; Amighi, K. Formulation and in vitro evaluation of highly dispersive insulin dry powder formulations for lung administration. Eur. J. Pharm. Biopharm. 2010, 76, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Salama, R.; Young, P.; Traini, D. Preparation and in vitro evaluation of salbutamol-loaded lipid microparticles for sustained release pulmonary therapy. J. Microencapsul. 2012, 29, 225–233. [Google Scholar] [CrossRef]
- Scalia, S.; Traini, D.; Young, P.M.; di Sabatino, M.; Passerini, N.; Albertini, B. Comparison of spray congealing and melt emulsification methods for the incorporation of the water-soluble salbutamol sulphate in lipid microparticles. Pharm. Dev. Technol. 2013, 18, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Trotta, V.; Traini, D.; Young, P.M.; Sticozzi, C.; Cervellati, F.; Valacchi, G. Incorporation of quercetin in respirable lipid microparticles: Effect on stability and cellular uptake on A549 pulmonary alveolar epithelial cells. Colloids Surf. B Biointerfaces 2013, 112, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Daman, Z.; Gilani, K.; Rouholamini Najafabadi, A.; Eftekhari, H.R.; Barghi, M.A. Formulation of inhalable lipid-based salbutamol sulfate microparticles by spray drying technique. DARU J. Pharm. Sci. 2014, 22, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignjatović, J.; Đuriš, J.; Cvijić, S.; Dobričić, V.; Montepietra, A.; Lombardi, C.; Ibrić, S.; Rossi, A. Development of solid lipid microparticles by melt-emulsification/spray-drying processes as carriers for pulmonary drug delivery. Eur. J. Pharm. Sci. 2021, 156, 105588. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Tougas, T.P.; Christopher, D.; Mitchell, J.P.; Strickland, H.; Wyka, B.; van Oort, M.; Lyapustina, S. Improved quality control metrics for cascade impaction measurements of orally inhaled drug products (OIPs). AAPS PharmSciTech 2009, 10, 1276–1285. [Google Scholar] [CrossRef] [Green Version]
- Mohan, M.; Lee, S.; Guo, C.; Peri, S.P.; Doub, W.H. Evaluation of abbreviated impactor measurements (AIM) and efficient data analysis (EDA) for dry powder inhalers (DPIs) against the full-resolution next generation impactor (NGI). AAPS PharmSciTech 2017, 18, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Guideline on the Requirements for Clinical Documentation for Orally Inhaled Products (Oip) Including the Requirements for Demonstration of Therapeutic Equivalence between Two Inhaled Products for Use in the Treatment of Asthma and Chronic Obstructive Pulm. 2009, pp. 1–16. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-requirements-clinical-documentation-orally-inhaled-products-oip-including-requirements_en.pdf (accessed on 29 July 2021).
- Kuribayashi, R.; Yamaguchi, T.; Sako, H.; Takishita, T.; Takagi, K. Bioequivalence Evaluations of Generic Dry Powder Inhaler Drug Products: Similarities and Differences between Japan, USA, and the European Union. Clin. Pharmacokinet. 2017, 56, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Sakagami, M.; Byron, P.R. Cascade impactor practice for a high dose dry powder inhaler at 90 L/min: NGI versus modified 6-Stage and 8-Stage ACI. J. Pharm Sci. 2009, 98, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Russell-Graham, D.; Cooper, A.; Stobbs, B.; McAulay, E.; Bogard, H.; Heath, V.; Monsallier, E. Further Evaluation of the Fast-Screening Impactor for Determining Fine Particle Fraction of Dry Powder Inhalers; Drug delivery to the lungs-21; The Aerosol Society: Edinburgh, UK, 2010; Volume 21, pp. 374–377. [Google Scholar]
- Taki, M.; Marriott, C.; Zeng, X.; Martin, G.P. Aerodynamic deposition of combination dry powder inhaler formulations in vitro: A comparison of three impactors. Int. J. Pharm. 2010, 388, 40–51. [Google Scholar] [CrossRef]
- Mohammed, H.; Roberts, D.L.; Copley, M.; Hammond, M.; Nichols, S.C.; Mitchell, J.P. Effect of sampling volume on dry powder inhaler (DPI)-emitted aerosol aerodynamic particle size distributions (APSDs) measured by the Next-Generation Pharmaceutical Impactor (NGI) and the Andersen eight-stage cascade impactor (ACI). AAPS PharmSciTech 2012, 13, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Nichols, S.C.; Mitchell, J.P.; Sandell, D.; Andersson, P.U.; Fischer, M.; Howald, M.; Pengilley, R.; Krüger, P. A Multi-laboratory in vitro study to compare data from abbreviated and pharmacopeial impactor measurements for orally inhaled products: A report of the European aerosol group (EPAG). AAPS PharmSciTech 2016, 17, 1383–1392. [Google Scholar] [CrossRef]
- Yoshida, H.; Kuwana, A.; Shibata, H.; Izutsu, K.; Goda, Y. Comparison of aerodynamic particle size distribution between a next generation impactor and a cascade impactor at a range of flow rates. AAPS PharmSciTech 2017, 18, 646–653. [Google Scholar] [CrossRef]
- Lin, Y.; Yoon, R.; Chang, K.; Britton, W.J.; Morales, S.; Kutter, E.; Li, J.; Chan, H. Inhalable combination powder formulations of phage and ciprofloxacin for P. aeruginosa respiratory infections. Eur. J. Pharm. Biopharm. 2019, 142, 543–552. [Google Scholar] [CrossRef]
- Omer, H.; Husein, N.; Hamadameen, H. Comparison between the next generation impactor and the twin glass impinge as model pulmonary drug delivery devices. Zanco J. Med. Sci. 2019, 23, 74–80. [Google Scholar] [CrossRef]
- Wong, W.; Fletcher, D.F.; Traini, D.; Chan, H.K.; Young, P.M. The use of computational approaches in inhaler development. Adv. Drug Deliv. Rev. 2012, 64, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Vulović, A.; Šušteršič, T.; Cvijić, S.; Ibrić, S.; Filipović, N. Coupled in silico platform: Computational fluid dynamics (CFD) and physiologically-based pharmacokinetic (PBPK) modelling. Eur. J. Pharm. Sci. 2018, 113, 171–184. [Google Scholar] [CrossRef]
- Coates, M.S.; Chan, H.; Fletcher, D.F.; Chiou, H. Influence of mouthpiece geometry on the aerosol delivery performance of a dry powder inhaler. Pharm. Res. 2007, 24, 1450–1456. [Google Scholar] [CrossRef]
- Coates, M.S.; Fletcher, D.F.; Chan, H.K.; Raper, J.A. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: Grid structure and mouthpiece length. J. Pharm. Sci. 2004, 93, 2863–2876. [Google Scholar] [CrossRef]
- Coates, M.S.; Chan, H.; Fletcher, D.F.; Raper, J.A. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 2: Air Inlet Size. J. Pharm. Sci. 2006, 95, 1382–1392. [Google Scholar] [CrossRef]
- Coates, M.S.; Chan, H.K.; Fletcher, D.F.; Raper, J.A. Influence of air flow on the performance of a dry powder inhaler using computational and experimental analyses. Pharm. Res. 2005, 22, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, J.; Alexopoulos, A.H.; Kiparissides, C. Flow and particle deposition in the Turbuhaler: A CFD simulation. Int. J. Pharm. 2013, 448, 205–213. [Google Scholar] [CrossRef]
- Donovan, M.J.; Kim, S.I.N.H.; Raman, V.; Smyth, H.D. Dry powder inhaler device influence on carrier particle performance. J. Pharm. Sci. 2012, 101, 1097–1107. [Google Scholar] [CrossRef]
- Tong, Z.B.; Zheng, B.; Yang, R.Y.; Yu, A.B.; Chan, H.K. CFD-DEM investigation of the dispersion mechanisms in commercial dry powder inhalers. Powder Technol. 2013, 240, 19–24. [Google Scholar] [CrossRef]
- Milenkovic, J.; Alexopoulo, A.H.; Kiparissides, C. Airflow and particle deposition in a dry powder inhaler: An integrated CFD approach. Adv. Intell. Syst. Comput. 2014, 256, 127–140. [Google Scholar] [CrossRef]
- Coates, M.S.; Fletcher, D.F.; Chan, H.K.; Raper, J.A. The role of capsule on the performance of a dry powder inhaler using computational and experimental analyses. Pharm. Res. 2005, 22, 923–932. [Google Scholar] [CrossRef] [Green Version]
- Tong, Z.B.; Adi, S.; Yang, R.Y.; Chan, H.K.; Yu, A.B. Numerical investigation of the de-agglomeration mechanisms of fine powders on mechanical impaction. J. Aerosol Sci. 2011, 42, 811–819. [Google Scholar] [CrossRef]
- De Boer, A.H.; Hagedoorn, P.; Woolhouse, R.; Wynn, E. Computational fluid dynamics (CFD) assisted performance evaluation of the TwincerTM disposable high-dose dry. J. Pharm. Pharmacol. 2012, 64, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Longest, P.W.; Son, Y.J.; Holbrook, L.; Hindle, M. Aerodynamic factors responsible for the deaggregation of carrier-free drug powders to form micrometer and submicrometer aerosols. Pharm. Res. 2013, 30, 1608–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behara, S.R.; Farkas, D.R.; Hindle, M.; Longest, P.W. Development of a high efficiency dry powder inhaler: Effects of capsule chamber design and inhaler surface modifications. Pharm. Res. 2014, 31, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Milenkovic, J.; Alexopoulos, A.H.; Kiparissides, C. Deposition and fine particle production during dynamic flow in a dry powder inhaler: A CFD approach. Int. J. Pharm. 2014, 461, 129–136. [Google Scholar] [CrossRef]
- Tong, Z.; Kamiya, H.; Yu, A.; Chan, H.K.; Yang, R. Multi-scale modelling of powder dispersion in a carrier-based inhalation system. Pharm. Res. 2015, 32, 2086–2096. [Google Scholar] [CrossRef]
- Nguyen, D.; Remmelgas, J.; Björn, I.N.; van Wachem, B.; Thalberg, K. Towards quantitative prediction of the performance of dry powder inhalers by multi-scale simulations and experiments. Int. J. Pharm. 2018, 547, 31–43. [Google Scholar] [CrossRef]
- Bass, K.; Farkas, D.; Longest, W. Optimizing aerosolization using computational fluid dynamics in a pediatric air-jet dry powder inhaler. AAPS PharmSciTech 2019, 20, 329. [Google Scholar] [CrossRef] [PubMed]
- Longest, W.; Farkas, D.; Bass, K.; Hindle, M. Use of computational fluid dynamics (CFD) dispersion parameters in the development of a new DPI actuated with low air volumes. Pharm. Res. 2019, 36, 110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Shui, S.; Leung, Y.; Gupta, R. Flow and particle modelling of dry powder inhalers: Methodologies, recent development and emerging applications. Pharmaceutics 2021, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- CATIA Student Edition. Available online: https://edu.3ds.com/en/software/catia-v5-student-edition (accessed on 10 October 2021).
- Ansys Software. Available online: https://www.ansys.com/ (accessed on 10 October 2021).
- Ansys Software. Available online: https://www.ansys.com/products/meshing (accessed on 10 October 2021).
- Ansys Software. Available online: https://www.ansys.com/products/fluids/ansys-fluent (accessed on 10 October 2021).
- Milenkovic, J.M. Airflow and Particle Deposition in a Dry Powder Inhaler. A CFD and Particle Computational Approach; Aristotle University of Thessaloniki: Thessaloniki, Greece, 2015; Available online: http://ikee.lib.auth.gr/record/136474/files/GRI-2015-14388.pdf?version=1 (accessed on 9 October 2021).
- Sommerfeld, M.; van Wachem, B.; Oliemans, R. Best Practice Guidelines for Computational Fluid Dynamics of Dispersed Multiphase Flows; ERCOFTAC European Research Community on Flow, Turbulence and Combustion: Brussels, Belgium, 2008. [Google Scholar]
- El-Batsh, H.; Haselbacher, H. Numerical investigation of the effect of ash particle deposition. In ASME Turbo Expo 2002: Power for Land, Sea, and Air; ASME: New York, NY, USA, 2002; Volume 3610, pp. 1035–1043. [Google Scholar] [CrossRef]
- Dahneke, B. Further measurements of the bouncing of small latex spheres. J. Colloid Interface Sci. 1975, 51, 58–65. [Google Scholar] [CrossRef]
- Brach, R.M.; Dunn, P.F. A mathematical model of the impact and adhesion of microsphers. Aerosol Sci. Technol. 1992, 16, 51–64. [Google Scholar] [CrossRef]
- El-Din, A.H.; Diab, A. Assessment of losses in annual energy production of wind turbines subjected to sand erosion. In Proceedings of the International Conference on Fluid Dynamics, Cairo, Egypt, 19–20 December 2016. [Google Scholar]
- Sommerfeld, M.; Schmalfuß, S. Numerical analysis of carrier particle motion in a dry powder inhaler. J. Fluids Eng. Trans. ASME 2016, 138, 041308. [Google Scholar] [CrossRef]
- Soltani, M.; Ahmadi, G. On particle adhesion and removal mechanisms in turbulent flows. J. Adhes. Sci. Technol. 1994, 8, 763–785. [Google Scholar] [CrossRef]
- Wang, H.C. Effects of inceptive motion on particle detachment from surfaces. Aerosol Sci. Technol. 1990, 13, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Christopher, J.D.; Dey, M.; Lyapustina, S.; Mitchell, J.P.; Tougas, T.P.; van Oort, M.; Strickland, H.; Wyka, B.; Zaidi, K. Generalized simplified approaches for mass median aerodynamic determination. Pharmacop. Forum 2010, 36, 812–823. [Google Scholar]
- Hupet, F.; Lambot, S.; Javaux, M.; Vanclooster, M. On the identification of macroscopic root water uptake parameters from soil water content observations. Water Resour. Res. 2002, 38, 36-1–36-14. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Nichols, S.C. Meeting report: Summary of abbreviated impactor measurement workshop organized by the european pharmaceutical aerosol group. Ther. Deliv. 2011, 2, 307–310. [Google Scholar] [CrossRef] [Green Version]
















| Formulation | Lipid (%) | Poloxamer 188 (%) | SS (%) | Trehalose Addition | High Shear Mixing Time (min) | High Shear Mixing Speed (rpm) | T Inlet (°C) | T Outlet (°C) | Feed Rate (mL/min) | Spraying Airflow Rate (L/h) | Wash |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 5.00 | 0.40 | 1.00 | No | 8.00 | 13,400 | 89 | 58 | 1.80 | 473 | No |
| F2 | 5.00 | 1.50 | 1.00 | No | 2.00 | 13,400 | 89 | 58 | 1.80 | 670 | No |
| F3 | 5.00 | 1.50 | 2.00 | Yes | 2.00 | 13,400 | 89 | 60 | 1.80 | 670 | Yes |
| F4 | 5.00 | 1.50 | 1.00 | Yes | 2.00 | 13,400 | 89 | 56 | 1.80 | 670 | No |
| F5 | 5.00 | 1.50 | 1.00 | No | 2.00 | 13,400 | 80 | 50 | 1.80 | 670 | Yes |
| Formulation | dv10 (µm) | dv50 (µm) | dv90 (µm) | Span | True Density (g/cm3) |
|---|---|---|---|---|---|
| F1 | 2.24 ± 0.04 | 4.42 ± 0.07 | 8.84 ± 0.37 | 1.492 ± 0.071 | 1.050 ± 0.001 |
| F2 | 2.43 ± 0.02 | 4.67 ± 0.03 | 8.51 ± 0.06 | 1.304 ± 0.003 | 1.060 ± 0.002 |
| F3 | 2.20 ± 0.05 | 4.03 ±0.06 | 7.10 ± 0.03 | 1.217 ± 0.025 | 1.170 ± 0.001 |
| F4 | 1.87 ± 0.01 | 5.13 ±0.09 | 12.21 ± 0.06 | 2.016 ± 0.034 | 1.230 ± 0.001 |
| F5 | 2.26 ± 0.02 | 3.94 ± 0.03 | 6.63 ± 0.04 | 1.110 ± 0.003 | 1.030 ± 0.002 |
| Parameter Name | Symbol | Value | Unit | Reference |
|---|---|---|---|---|
| Young’s modulus for the inhaler wall surface | Es | 4.1 × 109 | Pa | [43] |
| Young’s modulus for particle | Ep | 1 × 109 | Pa | [43] |
| Work of adhesion | WA | 0.039 | J/m2 | [65,66] |
| Poisson’s ratio for the inhaler wall surface | vs | 0.35 | / | [43] |
| Poisson’s ratio for particle | vp | 0.40 | / | [43] |
| Particle density | ρp | Taken from Table 2 | kg/m3 | / |
| Air density (at 1013.25 h Pa (abs) and 15 °C) | ρ | 1.225 | kg/m3 | [43] |
| Dynamic viscosity of fluid | µ | 1.7894 × 10−5 | N s/m2 | [43] |
| Correction factor for the near wall | f | 1.70 | / | [65,66] |
| Cunningham correction factor | Cu | 1 (for spherical particles) | / | [65,66] |
| Static coefficient of friction | ks | 0.50 | / | [65,66] |
| NGI ** | TSI ** | FSI ** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | NRMSE | R2 | RMSE | NRMSE | R2 | RMSE | NRMSE | |
| CFD-DPM * | 0.0847 | 5.643 | 0.067 | 0.0342 | 8.651 | 0.106 | 0.0055 | 7.531 | 0.093 |
| FSI * | 0.5064 | 5.041 | 0.060 | 0.7686 | 3.380 | 0.041 | / | / | / |
| TSI * | 0.8969 | 4.183 | 0.049 | / | / | / | / | / | / |
| R2 | RMSE | NRMSE | |
|---|---|---|---|
| MMAD | 0.0643 | 4.044 | 1.232 |
| GSD | 0.2215 | 1.020 | 0.465 |
| NGI ** | TSI ** | FSI ** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | NRMSE | R2 | RMSE | NRMSE | R2 | RMSE | NRMSE | |
| CFD-DPM * | 0.0789 | 14.178 | 0.525 | 0.0111 | 18.263 | 0.592 | 0.0219 | 19.227 | 0.587 |
| FSI * | 0.5948 | 6.723 | 0.249 | 0.5868 | 5.726 | 0.186 | / | / | / |
| TSI * | 0.9440 | 4.669 | 0.173 | / | / | / | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignjatović, J.; Šušteršič, T.; Bodić, A.; Cvijić, S.; Đuriš, J.; Rossi, A.; Dobričić, V.; Ibrić, S.; Filipović, N. Comparative Assessment of In Vitro and In Silico Methods for Aerodynamic Characterization of Powders for Inhalation. Pharmaceutics 2021, 13, 1831. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13111831
Ignjatović J, Šušteršič T, Bodić A, Cvijić S, Đuriš J, Rossi A, Dobričić V, Ibrić S, Filipović N. Comparative Assessment of In Vitro and In Silico Methods for Aerodynamic Characterization of Powders for Inhalation. Pharmaceutics. 2021; 13(11):1831. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13111831
Chicago/Turabian StyleIgnjatović, Jelisaveta, Tijana Šušteršič, Aleksandar Bodić, Sandra Cvijić, Jelena Đuriš, Alessandra Rossi, Vladimir Dobričić, Svetlana Ibrić, and Nenad Filipović. 2021. "Comparative Assessment of In Vitro and In Silico Methods for Aerodynamic Characterization of Powders for Inhalation" Pharmaceutics 13, no. 11: 1831. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13111831