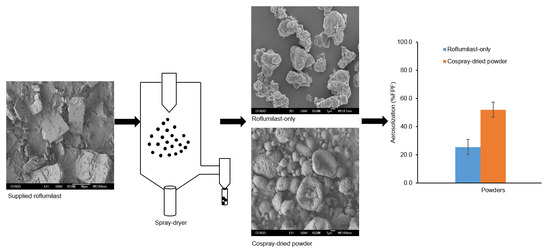
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive airflow obstruction associated with chronic lung inflammation, which impairs a patient’s quality of life, and acute exacerbation can lead to death [
1]. In contrast to other diseases, the morbidity and mortality rate of COPD continue to increase, and COPD is a major global health burden [
2]. Bronchodilators, corticosteroids, muscarinic antagonists, methylxanthines, phosphodiesterase-4 (PDE4) inhibitors, and mucolytic agents currently constitute the treatment for COPD [
3]. Among these, the PDE4 inhibitor shows particular promise against COPD as PDE enzymes hydrolyze and inactivate cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), which regulate smooth muscle relaxation and inflammatory mediator release [
4].
Roflumilast (3-cyclo-propylmethoxy-4-difluoromethoxy-
N-[3,5-dichloropyrid-4-yl]-benzamide) is a selective PDE4 inhibitor used to control acute exacerbations in patients with severe COPD [
3]. Although the current roflumilast oral therapy has a clear purpose in the prevention of acute exacerbations, it comes with undesired adverse effects including gastrointestinal disturbances, insomnia, and weight loss [
5,
6]. As COPD is marked by local inflammation, a formulation delivering the drug directly to the site of action could circumvent the associated side effects by reducing systemic exposure. Inhaled roflumilast was more effective than oral roflumilast in curbing and relieving allergen-induced air-flow obstructions in Brownian Norway rats when intratracheally administered [
7]. A spray-dried roflumilast dry powder formulation incorporating hydroxypropyl-β-cyclodextrin (HPβCD) has been reported [
8]. While HPβCD has been shown to be non-toxic upon inhalation, it was found to slightly increase lymphocyte count in the bronchoalveolar lavage, the implications of which remain unknown [
9]. The fine particle fraction (FPF) of the reported formulations was between 44 and 58% when dispersed from a Handihaler device at an inhalation flow rate of 60 L/min. A new formulation strategy could improve the FPF. In addition, the implications of using different devices and inspiratory flow rates on aerosol performance for such low-dose formulations have not been explored.
While formulations strategies are important for pulmonary drug delivery, the device used and the inspiratory flow rate are also of paramount importance [
10,
11,
12]. The currently available dry powder inhaler (DPI) devices can broadly be categorized into low (<5 Mbar), medium (5–10 Mbar), and high-resistance (>10 Mbar) devices based on the pressure drop [
11]. The Breezhaler and Aerolizer are examples of low-resistance devices. The Accuhaler, Clickhaler, Turbohaler, and Rotahaler are devices offering medium resistance, whereas high-resistance devices include the Handihaler and Easyhaler. Patients with COPD have compromised lung functions [
11,
13,
14]. Therefore, an intricate balance between inspiratory flow rate and device resistance must be considered to achieve the desired aerosol performance.
The aim of this study was to develop a highly dispersible low-dose dry powder formulation of roflumilast using spray drying. For this, trehalose was selected to form the bulk of the formulation. Trehalose is a non-reducing sugar that is easily cleared by metabolism and is non-toxic to the lungs [
15,
16]. To achieve good dispersion, L-leucine was incorporated into the formulation. Other amino acids are also used as aerosolization enhancers, but L-leucine is the most widely used amino acid and is generally regarded as safe (GRAS) for inhalation delivery [
15,
17,
18]. In addition to the formulation endeavors, the influence of device on aerosolization of the optimized formulation was also investigated. Three different devices (Aerolizer, Rotahaler, and Handihaler) were selected based on their resistance capacities. Moreover, the influence of the inhalation flow rate (30, 45, and 60 L/min) on aerosolization was examined. The cytotoxicity of the formulation on an alveolar cell line was also investigated.
2. Materials and Methods
2.1. Materials
Roflumilast and L-leucine were purchased from Hubei Yuancheng Technology Co. Ltd., Wuhan, China and Hangzhou Dayangchem Co., Ltd., Zhejiang, China, respectively. Acetonitrile, methanol, isopropanol (high-performance liquid chromatography, HPLC grade), and trehalose (analytical reagent, AR grade) were purchased from Merck, Germany. Ammonium acetate, glacial acetic acid (AR grade), and silicone oil (viscosity 10 cSt) were purchased from Sigma–Aldrich, St. Louis, MI, USA. Hard gelatin capsules (Size 3) were kindly donated by Capsugel Co., Ltd., Tokyo, Japan. Fresh Milli-Q water prepared using equipment from Millipore Corporation, Bedford, MA, USA was collected and filtered through 0.45 μm membrane filter manufactured by Phenomenex, CA, USA.
2.2. Quantification by HPLC Analysis
Roflumilast was analyzed using a validated reverse-phase HPLC method modified from Belal et al. [
19]. A reverse-phase HPLC system (Shimadzu, Kyoto, Japan) equipped with an LC-20AD solvent delivery unit, Prominence photodiode array (PDA) detector (Shimadzu SPD-M20A), degasser (Shimadzu DGU-20A5), and autosampler operated with Class-VP 7.4SP4 software was used. The mobile phase, ammonium acetate buffer (pH 6.3), acetonitrile, and methanol (30:35:35%
v/
v) was pumped at a 1.0 mL/min flow rate through a Synergi Fusion RP80A C18 column (4 µm, 150 × 4.6 mm; Phenomenex, Torrance, CA, USA) connected to a C18 security guard (Phenomenex Fusion RP, CA, USA; 4.0 × 3.0 mm). An aliquot (20 µL) of each sample was injected at ambient temperature and detected at a wavelength of 251 nm with a total run time of 8 min. The calibration curve (0.25–80 µg/mL) was linear (
R2 = 0.9999) with the limit of detection (LOD) and limit of quantitation (LOQ) of 0.04 and 0.11 µg/mL, respectively. The repeatability and reproducibility of the separately prepared quality control samples (10, 50, and 100 µg/mL) were within the acceptable limit (%Bias: ≤15; coefficient variation, %CV: ≤15).
2.3. Preparation of Powders, Study Design, and Optimization
A Buchi B-290 Mini Spray-Dryer (Buchi Labortechnik AG, Flawil, Switzerland) attached with a high-performance cyclone separator was used to produce the powder particles in a closed mode. Feed solutions were prepared in a co-solvent system of ethanol and water using nitrogen as an atomizing gas. The roflumilast was first dissolved in ethanol; trehalose and L-leucine were dissolved in water. Then, the two solutions were mixed and sonicated for 5 min to give the final desired feed concentration. Prepared feed solutions were spray-dried using the following constant variables: ratio of formulation components (roflumilast, L-leucine, and trehalose: 4, 24, and 72%
w/
w), co-solvent mixture (ethanol and water, 70:30%
v/
v), feed rate (2 mL/min), aspiration (100%), inlet temperature (165 °C), and nozzle diameter (0.7 mm) as well as independent variables: feed concentration and spray-gas flow rate (
Table 1). The feed concentration and spray-gas flow rate were chosen as independent variables since these are the most prominent factors affecting the droplet formation, which eventually affects the spray drying yield and particle size. The outlet temperature was recorded based on the inlet temperature.
A
full-factorial design study based on independent variables was undertaken to prepare the powder formulations (
Table 2) and investigate the effect of the independent variables on particle size and process yield. The main effects of the factors on particle size and yield were analyzed by balanced ANOVA using Minitab
TM software (Minitab Inc., Version 16, State College, PA, USA). Triplicate batches of the optimized powder formulation based on particle size (with maximum process yield) analysis were prepared to evaluate the inter-batch variability in aerosolization and related properties. The roflumilast-only powder was prepared using the optimized spray-drying conditions after dissolving roflumilast in the ethanol and water co-solvent system at a total solid content of 0.75%
w/
v. The spray-dried powders from the sample collector were transferred into screw-capped glass vials and stored at room temperature in a desiccator until evaluation. The following equation was used to calculate the percentage yield of the spray-dried powders.
2.4. Particle Size and Size Distribution by Laser Light Diffraction
The particle size distribution of the powders was determined using the LA-950 diffraction analyzer (Horiba Ltd., Kyoto, Japan). The samples were dispersed in isopropanol and transferred into a fraction cell for analysis. The refractive index of roflumilast (1.6) was used to measure the volumetric particle diameters. All experiments were done in triplicate for the optimized formulation. The span values were calculated using the following equation:
where
D90,
D50, and
D10 represent the equivalent diameters at 90%, 50%, and 10% cumulative volume of the particles, respectively.
2.5. Crystallinity by X-ray Powder Diffraction (XRPD)
The crystallinity of the powder samples was determined with an X’Pert PRO MPD PW3040/60 X-ray diffractometer (Malvern Panalytical, Malvern, UK) equipped with Cu Kα radiation. An aluminum holder was used to analyze the samples over a 2θ range of 5–35° at a rate of 2°/min. The PANalytical High Score software was used to analyze the diffractogram.
2.6. Residual Solvent Content by Thermogravimetric Analysis (TGA)
The residual solvent content of the formulations was determined using a Q50 thermogravimetric analyzer (TA Instruments, New Castle, DE, USA). Approximately 5 mg of each sample was placed in a platinum pan and heated at a rate of 10 °C/min from room temperature (≈25 °C) to 150 °C under a nitrogen purge. The weight change was analyzed using the TRIOS software (TA Instruments, New Castle, DE, USA).
2.7. Phase Transition by Differential Scanning Calorimetry (DSC)
The thermal behavior and phase transition of the supplied and spray-dried powders were investigated using a Q100 DSC system (TA Instruments, New Castle, DE, USA) calibrated with indium. Approximately 5 mg of powder sample was placed into an aluminum DSC pan and sealed with an aluminum lid (TA instruments) using a manual pan crimper press (PerkinElmer, Boston, MA, USA). An empty aluminum pan sealed with a lid was used as a reference pan. The DSC pans were scanned at 5 °C/min over the range of 0 to 350 °C. During heating, nitrogen was used as a purging gas (at 50 mL/min).
2.8. Powder Density and Flow Property
A 1 mL tuberculin syringe was used to determine the powder density. The bulk volume was measured after adding powder into the syringe up to 0.5 mL mark, and the weight of the powder sample was used to calculate the bulk density before tapping. Approximately after 100 taps/min (until no volume change), the volume was observed (tapped volume) and used to calculate the tapped density from powder volume. Both bulk and tapped densities were used to calculate the flow property of the powders using the following equation:
2.9. Particle Morphology by Scanning Electron Microscopy (SEM)
The surface morphologies of the powders were examined using a JEOL 6700F scanning electron microscope (JEOL Ltd., Tokyo, Japan). A K575X sputter coater (EM Technologies Ltd., Kent, England) was used to sputter-coat the particles on carbon sticky tape with a gold/palladium alloy. The images were generated at 5 kV acceleration voltage.
2.10. In Vitro Aerosolization Performance by Next-Generation Impactor (NGI)
The Next-Generation Impactor (NGI) from Copley Scientific Ltd., Nottingham, UK, was used to determine the in vitro aerosolization performances of the spray-dried powder formulations. A Copley TPK 2000 critical flow controller (Copley Scientific Ltd., Nottingham, UK) and an electronic digital flow meter (Copley Scientific Model DFM2000) were used to achieve the desired flow rate. Approximately 20 mg of the powder was filled into gelatin capsules (Capsugel Japan Inc., Kanagawa, Japan) and dispersed using an Aerolizer at a flow rate of 100 L/min over 2.4 s to inspire 4 L air. This volume (4 L) is considered as the normal forced inhalation capacity of an average-sized male. This 100 L/min flow rate was chosen since we used Aerolizer, which is a low-resistance device (pressure drop < 5 Mbar) [
20]. Approximately 15 mL of water was placed in the pre-separator of the device, whereas the stages 1 to 7 (S1 to S7) and Micro-Orifice Collector (MOC) were coated with a layer of silicone oil (viscosity = 10
−5 m
2/s at 25 °C). The cut-off diameters for NGI stages 2–7 at 100 L/min flow rate were 3.42, 2.18, 1.31, 0.72, 0.40, and 0.24 μm, respectively. Following actuation, the dispersed powders retained on the device along with the capsule and the powder deposited in all the parts of NGI were collected using acetonitrile and methanol mixture at a ratio of 10:90%
v/
v and analyzed by the HPLC method. Triplicate samples were prepared for the in vitro aerosolization performances of each formulation. All the aerosolization performances were conducted at room conditions (20 ± 2 °C temperature and 32 ± 2% relative humidity (RH)).
The aerodynamic parameters (recovered dose, emitted dose (ED), fine particle dose (FPD), fine particle fraction (FPF), mass median aerodynamic diameter (MMAD), and geometric standard deviation (GSD) were calculated for each run. The recovered dose (RD) was the total amount of drug retained on the device and deposited in different stages of the NGI, including MOC. The ED was the RD minus drug retained on the device. The FPD was the amount of drug deposited in stages from S2 to MOC. The FPF (%) was FPD expressed relative to ED. %ED was expressed relative to RD. The MMAD and GSD were calculated using the CITDAS software (Copley Scientific Ltd., Nottingham, UK).
2.11. Stability Studies
A single batch of roflumilast-only and optimized cospray-dried powder was stored in an open Petri dish at two different RH conditions (15% and 75%) at 25 ± 2 °C for one month to investigate the effect of low and high humidity on aerosolization performance. After storage, the aerosolization performance and other physicochemical properties (morphology, crystallinity, and drug content) of the powders were evaluated by the methods mentioned above.
2.12. Effect of Inhaler Device and Flow Rate on Aerosolization
The influence of inhaler devices and airflow rates on the aerosolization performances of the powder formulations were examined using three different inhaler devices (Aerolizer, Rotahaler, and Handihaler) at three different flow rates (30, 45, and 60 L/min). Devices were selected based on their resistance (Aerolizer (pressure drop < 5 Mbar), Rotahaler (pressure drop 5–10 Mbar), and Handihaler (pressure drop > 10 Mbar) to airflow. Aerolizer (Novartis Pharmaceuticals UK Ltd., London, UK), Rotahaler (GlaxoSmithKline, London, UK), and Handihaler (Boehringer-Ingelheim, Ingelheim am Rhein, Germany) are low, medium, and high resistance devices, respectively [
10,
21]. The in vitro aerosolization was performed following the same procedure mentioned above. Triplicate experiments were carried out for each device at each flow rate.
2.13. Cytotoxicity Studies by MTT Assay
The cytotoxicity of the roflumilast-only and cospray-dried roflumilast formulations was conducted using MTT assay on A549 (alveolar basal epithelium) cell line (ATCC, MD, USA). The A549 cell line was cultured in F12K medium. The medium was supplemented with 10% (
v/
v) fetal bovine serum and 1% penicillin solution. The cells were incubated in 5% carbon dioxide and 95% relative humidity at 37 °C. Further seeding was done in microtiter plates at a density of 5 × 104 cells/well and allowed to stand overnight for cell adhesion. Then, the cells were treated with 0.5, 1, and 4 µg/mL of roflumilast-only with a final volume of 200 µL (
n = 3 at each concentration) for each cell line. Similarly, 12.5, 25, and 100 µg/mL of cospray-dried formulation was used to be equivalent to the roflumilast-only powder concentrations of 0.5, 1, and 4 µg/mL, respectively. The concentrations were chosen based on low aqueous solubility (≈5 µg/mL) of roflumilast [
22]. The cell viability (cytotoxicity) was calculated using the following formula:
2.14. Statistical Analysis
All data were expressed as mean ± standard deviation. The inter-batch variability and intra-batch variability for aerosolization were compared using the nested ANOVA. Subsequent statistical analyses were performed by one-way analysis of variance (ANOVA) with the Student–Newman–Keuls test (compare all pairs) as a post hoc test at (p < 0.05). Instat Graphpad Prism software (version 4.00; GraphPad Software, San Diego, CA, USA) and Minitab (version 16; Minitab Inc., State College, PA, USA) were used for statistical analysis. Mean, standard deviation, and percentage relative standard deviation (%RSD) were calculated using MS Excel.