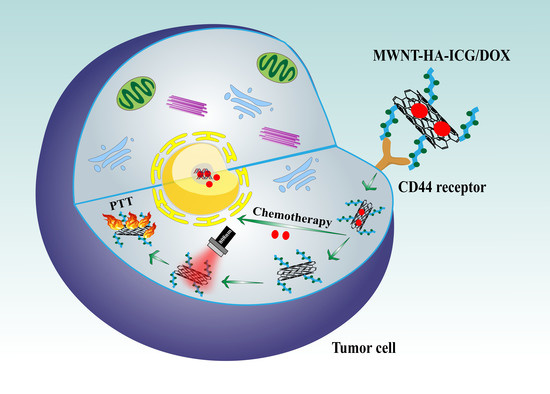
NIR Light-Triggered Chemo-Phototherapy by ICG Functionalized MWNTs for Synergistic Tumor-Targeted Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Cell Lines and Animals
2.2. Preparation of MWNT-HA-ICG/DOX Nanocomplexes
2.2.1. Carboxylation of MWNTs
2.2.2. Synthesis of HA-ICG
2.2.3. Synthesis of MWNT-HA-ICG
2.2.4. Synthesis of MWNT-HA-ICG/DOX
2.3. Characterization of MWNT-Based Formulations
2.4. In Vitro Release of DOX from MWNT-HA-ICG/DOX
2.5. In Vitro Photothermal Effect of MWNT-Based Formulations
2.6. In Vitro Cytotoxicity Assay
2.7. Cellular Uptake and Intracellular Trafficking
2.8. Cell Apoptosis Assessment
2.9. In Vivo Imaging Study
2.10. In Vivo Antitumor Efficacy
2.11. Statistical Analysis
3. Results
3.1. Synthesis of MWNT-HA-ICG/DOX
3.2. Characterization of MWNT-HA-ICG/DOX
3.3. Particle Size, Zeta Potential, and Morphology
3.4. In Vitro Drug Release and Photothermal Effect of MWNT-Based Formulations
3.5. In Vitro Cytotoxicity Studies
3.6. In Vitro Cellular Uptake Studies
3.7. Intracellular Distribution and Cell Apoptosis Studies
3.8. In Vivo Targeting Study
3.9. In Vivo Antitumor Efficacy Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, K.; Cao, X.; Li, M.; Su, Y.; Li, H.; Xie, M.; Zhang, Z.; Gao, H.; Xu, X.; Han, Y.; et al. A TRAIL-Delivered Lipoprotein-Bioinspired Nanovector Engineering Stem Cell-Based Platform for Inhibition of Lung Metastasis of Melanoma. Theranostics 2019, 9, 2984–2998. [Google Scholar] [CrossRef]
- Su, Y.J.; Wang, T.T.; Su, Y.N.; Li, M.; Zhou, J.P.; Zhang, W.; Wang, W. A neutrophil membrane-functionalized black phosphorus riding inflammatory signal for positive feedback and multimode cancer therapy. Mater. Horiz. 2020, 7, 574–585. [Google Scholar] [CrossRef]
- Li, M.; Su, Y.; Zhang, F.; Chen, K.; Xu, X.; Xu, L.; Zhou, J.; Wang, W. A dual-targeting reconstituted high density lipoprotein leveraging the synergy of sorafenib and antimiRNA21 for enhanced hepatocellular carcinoma therapy. Acta Biomater. 2018, 75, 413–426. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Li, J.; Zhao, Q.; Pan, T.; Zhong, H.; Wang, W. Advanced and Innovative Nano-Systems for Anticancer Targeted Drug Delivery. Pharmaceutics 2021, 13, 1151. [Google Scholar] [CrossRef]
- Tang, L.; He, S.; Yin, Y.; Liu, H.; Hu, J.; Cheng, J.; Wang, W. Combination of Nanomaterials in Cell-Based Drug Delivery Systems for Cancer Treatment. Pharmaceutics 2021, 13, 1888. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Zhou, J.; Gu, X.; Wang, W.; Liu, C.; Bao, X.; Wang, C.; Li, Y.; Zhang, Q. Direct cytosolic siRNA delivery by reconstituted high density lipoprotein for target-specific therapy of tumor angiogenesis. Biomaterials 2014, 35, 7214–7227. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Naghinejad, M.; Kasaeian, A.; Lohrasbi, A.; Shahraeini, S.S.; Zomorodbakhsh, S. The Applications of Carbon Nanotubes in the Diagnosis and Treatment of Lung Cancer: A Critical Review. Int. J. Nanomed. 2020, 15, 7063–7078. [Google Scholar] [CrossRef]
- Liu, X.; Ying, Y.; Ping, J. Structure, synthesis, and sensing applications of single-walled carbon nanohorns. Biosens. Bioelectron. 2020, 167, 112495. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Frank, A.L. Awareness: Potential toxicities of carbon nanotubes. Transl. Lung Cancer Res. 2019, 8 (Suppl. 4), S471–S472. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Jeon, J.Y.; Ha, T.J. Carbon nanotubes: An effective platform for biomedical electronics. Biosens. Bioelectron. 2020, 150, 111919. [Google Scholar] [CrossRef]
- Costa, P.M.; Bourgognon, M.; Wang, J.T.; Al-Jamal, K.T. Functionalised carbon nanotubes: From intracellular uptake and cell-related toxicity to systemic brain delivery. J. Control. Release 2016, 241, 200–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garriga, R.; Herrero-Continente, T.; Palos, M.; Cebolla, V.L.; Osada, J.; Munoz, E.; Rodriguez-Yoldi, M.J. Toxicity of Carbon Nanomaterials and Their Potential Application as Drug Delivery Systems: In Vitro Studies in Caco-2 and MCF-7 Cell Lines. Nanomaterials 2020, 10, 1617. [Google Scholar] [CrossRef]
- Corletto, A.; Shapter, J.G. Nanoscale Patterning of Carbon Nanotubes: Techniques, Applications, and Future. Adv. Sci. (Weinh) 2020, 8, 2001778. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black Phosphorus Nanosheets Immobilizing Ce6 for Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, Y.; Xu, X.; Zhou, J.; Xu, L.; Xu, X.; Wang, D.; Li, M.; Chen, K.; Wang, W. On-Demand Versatile Prodrug Nanomicelle for Tumor-Specific Bioimaging and Photothermal-Chemo Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 38700–38714. [Google Scholar] [CrossRef]
- Xu, X.; Lu, H.; Lee, R. Near Infrared Light Triggered Photo/Immuno-Therapy Toward Cancers. Front. Bioeng. Biotechnol. 2020, 8, 488. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Mei, L.; Fan, R.; Wang, Y.; Nie, C.; Tong, A.; Guo, G. Facile construction of targeted pH-responsive DNA-conjugated gold nanoparticles for synergistic photothermal-chemotherapy. Chin. Chem. Lett. 2021, 32, 1775–1779. [Google Scholar] [CrossRef]
- Shang, T.; Yu, X.; Han, S.; Yang, B. Nanomedicine-based tumor photothermal therapy synergized immunotherapy. Biomater. Sci. 2020, 8, 5241–5259. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active Targeting of Orthotopic Glioma Using Biomimetic Proteolipid Nanoparticles. ACS Nano 2019, 13, 386–398. [Google Scholar] [CrossRef]
- Ding, K.; Zheng, C.; Sun, L.; Liu, X.; Yin, Y.; Wang, L. NIR light-induced tumor phototherapy using ICG delivery system based on platelet-membrane-camouflaged hollow bismuth selenide nanoparticles. Chin. Chem. Lett. 2020, 31, 1168–1172. [Google Scholar] [CrossRef]
- Porcu, E.P.; Salis, A.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol. Adv. 2016, 34, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hu, Y.; Wang, Y.; Xu, X.; Yuan, Y.; Li, Y.; Wang, Z.; Chen, K.; Zhang, F.; Ding, X.; et al. A precision-guided MWNT mediated reawakening the sunk synergy in RAS for anti-angiogenesis lung cancer therapy. Biomaterials 2017, 139, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, J.; Zhang, W.; Zhao, Y.; Zhang, S.; Zhang, S. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydr. Polym. 2021, 251, 117103. [Google Scholar] [CrossRef]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Li, W.; Zhou, C.; Fu, Y.; Chen, T.; Liu, X.; Zhang, Z.; Gong, T. Targeted delivery of hyaluronic acid nanomicelles to hepatic stellate cells in hepatic fibrosis rats. Acta Pharm. Sin. B 2020, 10, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.; El-Bakly, W.; Sobhy, A.; Wagdy, O.; Reda, A.; Aboelenin, O.; Marzouk, A.; El Habak, K.; Mostafa, R.; Ali, M.A.; et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed. Pharmacother. 2017, 95, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control. Release 2020, 328, 171–191. [Google Scholar] [CrossRef]
- Huang, G.; Chen, J. Preparation and applications of hyaluronic acid and its derivatives. Int. J. Biol. Macromol. 2019, 125, 478–484. [Google Scholar] [CrossRef]
- Singh, P.; Wu, L.; Ren, X.; Zhang, W.; Tang, Y.; Chen, Y.; Carrier, A.; Zhang, X.; Zhang, J. Hyaluronic-acid-based beta-cyclodextrin grafted copolymers as biocompatible supramolecular hosts to enhance the water solubility of tocopherol. Int. J. Pharm. 2020, 586, 119542. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, M.; Zhang, Z.; Cui, C.; Zhou, J.; Yin, L.; Lv, H. Design, synthesis and evaluation of multi-functional tLyP-1-hyaluronic acid-paclitaxel conjugate endowed with broad anticancer scope. Carbohydr. Polym. 2017, 156, 97–107. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Ding, Y.; Li, J.; Li, M.; Liang, X.; Zhou, J.; Wang, W. Biomimetic HDL nanoparticle mediated tumor targeted delivery of indocyanine green for enhanced photodynamic therapy. Colloids Surf. B Biointerfaces 2016, 148, 533–540. [Google Scholar] [CrossRef]
- Toth, D.; Hailegnaw, B.; Richheimer, F.; Castro, F.A.; Kienberger, F.; Scharber, M.C.; Wood, S.; Gramse, G. Nanoscale Charge Accumulation and Its Effect on Carrier Dynamics in Tri-cation Perovskite Structures. ACS Appl. Mater. Interfaces 2020, 12, 48057–48066. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Smith, B.D. Deuterated Indocyanine Green (ICG) with Extended Aqueous Storage Shelf-Life: Chemical and Clinical Implications. Chemistry 2021, 27, 14535–14542. [Google Scholar] [CrossRef]
- Della Pelle, G.; Delgado Lopez, A.; Salord Fiol, M.; Kostevsek, N. Cyanine Dyes for Photo-Thermal Therapy: A Comparison of Synthetic Liposomes and Natural Erythrocyte-Based Carriers. Int. J. Mol. Sci 2021, 22, 6914. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. ICG-Loaded PEGylated BSA-Silver Nanoparticles for Effective Photothermal Cancer Therapy. Int. J. Nanomed. 2020, 15, 5459–5471. [Google Scholar] [CrossRef]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-step assembly of DOX/ICG loaded lipid--polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef]
- Li, C.; Guan, H.; Li, Z.; Wang, F.; Wu, J.; Zhang, B. Study on different particle sizes of DOX-loaded mixed micelles for cancer therapy. Colloids Surf. B Biointerfaces 2020, 196, 111303. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Zhang, A.; Mei, Y.; Xiao, Q.; Xu, X.; Wang, W. NIR Light-Triggered Chemo-Phototherapy by ICG Functionalized MWNTs for Synergistic Tumor-Targeted Delivery. Pharmaceutics 2021, 13, 2145. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122145
Tang L, Zhang A, Mei Y, Xiao Q, Xu X, Wang W. NIR Light-Triggered Chemo-Phototherapy by ICG Functionalized MWNTs for Synergistic Tumor-Targeted Delivery. Pharmaceutics. 2021; 13(12):2145. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122145
Chicago/Turabian StyleTang, Lu, Aining Zhang, Yijun Mei, Qiaqia Xiao, Xiangting Xu, and Wei Wang. 2021. "NIR Light-Triggered Chemo-Phototherapy by ICG Functionalized MWNTs for Synergistic Tumor-Targeted Delivery" Pharmaceutics 13, no. 12: 2145. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13122145