Dapagliflozin Mitigates Hypotension in Lipopolysaccharide-Induced Acute Inflammation Independent of Glycemia Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diabetes Induction
2.3. Treatment and LPS-Induced Sepsis
2.4. Blood Pressure and Heart Rate Measurement
2.5. Histopathology
2.6. Cytokine Analysis
2.7. Western Blotting
2.8. Nitric Oxide Assay
2.9. Statistical Analysis
3. Results
3.1. DAPA Regulates FBG Levels, Water/Food Intake, Weight Gain, and Urine Flow in Diabetic Rats
3.2. DAPA Regulates Blood Pressure in Diabetic Rats
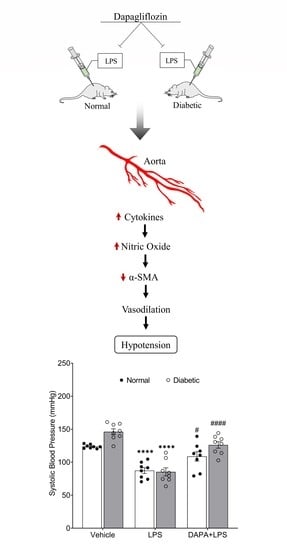
3.3. DAPA Attenuates LPS-Induced Vasodilation and Hypotension
3.4. DAPA Prevents LPS-Induced Cytokines Production
3.5. DAPA Regulates Histological Changes, Nitric Oxide Production and Expression of iNOS and α-SMA in Aortas of Septic Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.H.; Li, X.; Delozier, N.L.; Toto, R.D.; Moe, O.W.; Yee, J.; Neyra, J.A. Sepsis-Associated Acute Kidney Disease and Long-term Kidney Outcomes. Kidney Med. 2021, 3, 507–514.e1. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 2050312119835043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishmael, L.; Zalocha, J. ST-Elevation Myocardial Infarction in the Presence of Septic Shock. Case Rep. Crit. Care 2020, 2020, 8879878. [Google Scholar] [CrossRef]
- Castardo-de-Paula, J.C.; de Campos, B.H.; de Jager, L.; Amorim, E.D.T.; Zanluqui, N.G.; de Farias, C.C.; Higachi, L.; Pinge-Filho, P.; Barbosa, D.S.; Martins-Pinge, M.C. Effects of Inducible Nitric Oxide Synthase Inhibition on Cardiovascular Risk of Adult Endotoxemic Female Rats: Role of Estrogen. Front. Physiol. 2018, 9, 1020. [Google Scholar] [CrossRef]
- Lin, T.L.; Shu, C.C.; Chen, Y.M.; Lu, J.J.; Wu, T.S.; Lai, W.F.; Tzeng, C.M.; Lai, H.C.; Lu, C.C. Like Cures Like: Pharmacological Activity of Anti-Inflammatory Lipopolysaccharides from Gut Microbiome. Front. Pharmacol. 2020, 11, 554. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Tan, S.; Long, Z.; Hou, X.; Lin, Y.; Xu, J.; You, X.; Wang, T.; Zhang, Y. H2 Protects Against Lipopolysaccharide-Induced Cardiac Dysfunction via Blocking TLR4-Mediated Cytokines Expression. Front. Pharmacol. 2020, 10, 865. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.J.; Shih, C.C.; Chang, K.Y.; Liao, M.H.; Liaw, W.J.; Wu, C.C.; Tsao, C.M. Angiotensin-(1-7) treatment blocks lipopolysaccharide-induced organ damage, platelet dysfunction, and IL-6 and nitric oxide production in rats. Sci. Rep. 2021, 11, 610. [Google Scholar] [CrossRef]
- Costantini, E.; Carlin, M.; Porta, M.; Brizzi, M.F. Type 2 diabetes mellitus and sepsis: State of the art, certainties and missing evidence. Acta Diabetol. 2021, 58, 1139–1151. [Google Scholar] [CrossRef]
- Schuetz, P.; Castro, P.; Shapiro, N.I. Diabetes and sepsis: Preclinical findings and clinical relevance. Diabetes Care 2011, 34, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frydrych, L.M.; Fattahi, F.; He, K.; Ward, P.A.; Delano, M.J. Diabetes and Sepsis: Risk, Recurrence, and Ruination. Front. Endocrinol. 2017, 8, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi-Marjaneh, R.; Paseban, M.; Sahebkar, A. Natural products with SGLT2 inhibitory activity: Possibilities of application for the treatment of diabetes. Phytother. Res. 2019, 33, 2518–2530. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H. Role of SGLT2 Inhibitors in Patients with Diabetes Mellitus and Heart Failure. Curr. Heart Fail. Rep. 2017, 14, 275–283. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Chin, K.L.; Ofori-Asenso, R.; Hopper, I.; von Lueder, T.G.; Reid, C.M.; Zoungas, S.; Wang, B.H.; Liew, D. Potential mechanisms underlying the cardiovascular benefits of sodium glucose cotransporter 2 inhibitors: A systematic review of data from preclinical studies. Cardiovasc. Res. 2019, 115, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef] [Green Version]
- Ahmed-Sarwar, N.; Nagel, A.K.; Leistman, S.; Heacock, K. SGLT-2 Inhibitors: Is There a Role in Type 1 Diabetes Mellitus Management? Ann. Pharmacother. 2017, 51, 791–796. [Google Scholar] [CrossRef]
- Patel, D.K.; Strong, J. The Pleiotropic Effects of Sodium-Glucose Cotransporter-2 Inhibitors: Beyond the Glycemic Benefit. Diabetes Ther. 2019, 10, 1771–1792. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Postmus, D.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Held, C.; Hou, F.F.; et al. Effect of Dapagliflozin on Clinical Outcomes in Patients with Chronic Kidney Disease, With and Without Cardiovascular Disease. Circulation 2021, 143, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, D.; De Angelis, A.; Ciuffreda, L.P.; Coppini, R.; Cozzolino, A.; Miccichè, A.; Dell’Aversana, C.; D’Amario, D.; Cianflone, E.; Scavone, C.; et al. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol. Res. 2020, 157, 104781. [Google Scholar] [CrossRef]
- Rad, M.G.; Sharifi, M.; Meamar, R.; Soltani, N. The role of pancreas to improve hyperglycemia in STZ-induced diabetic rats by thiamine disulfide. Nutr. Diabetes 2022, 12, 32. [Google Scholar] [CrossRef]
- Ilçe, F.; Gök, G.; Pandir, D. Acute effects of lipopolysaccharide (LPS) in kidney of rats and preventive role of vitamin E and sodium selenite. Hum. Exp. Toxicol. 2019, 38, 547–560. [Google Scholar] [CrossRef]
- Liu, K.F.; Niu, C.S.; Tsai, J.C.; Yang, C.L.; Peng, W.H.; Niu, H.S. Comparison of area under the curve in various models of diabetic rats receiving. Arch. Med. Sci. 2020, 18, 1078–1087. [Google Scholar] [CrossRef]
- Daugherty, A.; Rateri, D.; Hong, L.; Balakrishnan, A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J. Vis. Exp. 2009, 27, 1291. [Google Scholar] [CrossRef] [Green Version]
- Alanazi, W.A.; Alanazi, A.S.; El-Nagar, D.M.; Aljuraybah, A.M.; Alsanea, S.; Alharbi, M. Mechanism Underlying Triple VEGFR Inhibitor Tivozanib-Induced Hypertension in Mice Model. Pharmaceuticals 2023, 16, 295. [Google Scholar] [CrossRef]
- van Ruiten, C.C.; Smits, M.M.; Kok, M.D.; Serné, E.H.; van Raalte, D.H.; Kramer, M.H.H.; Nieuwdorp, M.; IJzerman, R.G. Mechanisms underlying the blood pressure lowering effects of dapagliflozin, exenatide, and their combination in people with type 2 diabetes: A secondary analysis of a randomized trial. Cardiovasc. Diabetol. 2022, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Batra, M.; Green, K.; Hejna, J.; Abuaysheh, S.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Dapagliflozin reduces systolic blood pressure and modulates vasoactive factors. Diabetes Obes. Metab. 2021, 23, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Requena-Ibáñez, J.A.; Picatoste, B.; Fardman, B.; Ishikawa, K.; Mazurek, R.; Pieper, M.; Sartori, S.; Rodriguez-Capitán, J.; Fuster, V.; et al. Cardioprotective Effect of Empagliflozin and Circulating Ketone Bodies during Acute Myocardial Infarction. Circ. Circ. Cardiovasc. Imaging 2023, 16, e015298. [Google Scholar] [CrossRef] [PubMed]
- Preau, S.; Vodovar, D.; Jung, B.; Lancel, S.; Zafrani, L.; Flatres, A.; Oualha, M.; Voiriot, G.; Jouan, Y.; Joffre, J.; et al. Correction to: Energetic dysfunction in sepsis: A narrative review. Ann. Intensive Care 2021, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Mayr, M.; Badimon, J. SGLT2 Inhibitors in Heart Failure: Targeted Metabolomics and Energetic Metabolism. Circulation 2022, 146, 819–821. [Google Scholar] [CrossRef]
- Lew, W.Y.; Bayna, E.; Molle, E.D.; Dalton, N.D.; Lai, N.C.; Bhargava, V.; Mendiola, V.; Clopton, P.; Tang, T. Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS ONE 2013, 8, e61057. [Google Scholar] [CrossRef] [Green Version]
- Al-Saffar, H.; Lewis, K.; Liu, E.; Schober, A.; Corrigan, J.J.; Shibata, K.; Steiner, A.A. Lipopolysaccharide-induced hypothermia and hypotension are associated with inflammatory signaling that is triggered outside the brain. Brain Behav. Immun. 2013, 28, 188–195. [Google Scholar] [CrossRef]
- Yoon, Y.D.; Lee, M.Y.; Choi, B.J.; Lee, C.W.; Lee, H.; Kwon, J.H.; Yang, J.W.; Kang, J.S. Protection against Lipopolysaccharide-Induced Endotoxemia by Terrein Is Mediated by Blocking Interleukin-1β and Interleukin-6 Production. Pharmaceuticals 2022, 15, 1429. [Google Scholar] [CrossRef]
- Liu, S.F.; Malik, A.B. NF-κB activation as a pathological mechanism of septic shock and inflammation. American journal of physiology. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L622–L645. [Google Scholar] [CrossRef]
- Chi, P.J.; Lee, C.J.; Hsieh, Y.J.; Lu, C.W.; Hsu, B.G. Dapagliflozin Ameliorates Lipopolysaccharide Related Acute Kidney Injury in Mice with Streptozotocin-induced Diabetes Mellitus. Int. J. Med. Sci. 2022, 19, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M.; Salama, S.A. Effect of taxifolin/dapagliflozin combination on colistin-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2021, 40, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, E.; Keyhanfar, F.; Delbandi, A.A.; Falak, R.; Hajimiresmaiel, S.J.; Shafiei, M. Dapagliflozin exerts anti-inflammatory effects via inhibition of LPS-induced TLR-4 overexpression and NF-κB activation in human endothelial cells and differentiated macrophages. Eur. J. Pharmacol. 2022, 918, 174715. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, Y.; Tsuchiya, K.; Shiba, K.; Mori, K.; Komiya, C.; Ogasawara, N.; Ogawa, Y. A reduced M1-like/M2-like ratio of macrophages in healthy adipose tissue expansion during SGLT2 inhibition. Sci. Rep. 2018, 8, 16113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Kim, S.A.; Choi, Y.A.; Park, D.Y.; Lee, J. Alpha-Smooth Muscle Actin-Positive Perivascular Cells in Diabetic Retina and Choroid. Int. J. Mol. Sci. 2020, 21, 2158. [Google Scholar] [CrossRef] [Green Version]
- Hallemeesch, M.M.; Janssen, B.J.; de Jonge, W.J.; Soeters, P.B.; Lamers, W.H.; Deutz, N.E. NO production by cNOS and iNOS reflects blood pressure changes in LPS-challenged mice. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E871–E875. [Google Scholar] [CrossRef]
- Chuaiphichai, S.; Starr, A.; Nandi, M.; Channon, K.M.; McNeill, E. Endothelial cell tetrahydrobiopterin deficiency attenuates LPS-induced vascular dysfunction and hypotension. Vasc. Pharmacol. 2016, 77, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.J.; Park, K.C.; Tokar, S.; Eykyn, T.R.; Fuller, W.; Pavlovic, D.; Swietach, P.; Shattock, M.J. SGLT2 inhibitors and the cardiac Na+/H+ exchanger-1: The plot thickens. Cardiovasc. Res. 2021, 117, 2702–2704. [Google Scholar] [CrossRef]
- Al-Shamasi, A.A.; Elkaffash, R.; Mohamed, M.; Rayan, M.; Al-Khater, D.; Gadeau, A.P.; Ahmed, R.; Hasan, A.; Eldassouki, H.; Yalcin, H.C.; et al. Crosstalk between Sodium-Glucose Cotransporter Inhibitors and Sodium-Hydrogen Exchanger 1 and 3 in Cardiometabolic Diseases. Int. J. Mol. Sci. 2021, 22, 12677. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, W.A.; Alharbi, T.; El-Nagar, D.M.; Albogami, A.M.; Alswayyed, M. Dapagliflozin Mitigates Hypotension in Lipopolysaccharide-Induced Acute Inflammation Independent of Glycemia Level. Pharmaceutics 2023, 15, 1683. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15061683
Alanazi WA, Alharbi T, El-Nagar DM, Albogami AM, Alswayyed M. Dapagliflozin Mitigates Hypotension in Lipopolysaccharide-Induced Acute Inflammation Independent of Glycemia Level. Pharmaceutics. 2023; 15(6):1683. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15061683
Chicago/Turabian StyleAlanazi, Wael A., Turki Alharbi, Doaa M. El-Nagar, Abdullah M. Albogami, and Mohammed Alswayyed. 2023. "Dapagliflozin Mitigates Hypotension in Lipopolysaccharide-Induced Acute Inflammation Independent of Glycemia Level" Pharmaceutics 15, no. 6: 1683. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15061683