1. Introduction
Estimation of plant traits using remote sensing data, such as leaf nitrogen concentration, leaf chlorophyll concentration (LChl) and leaf area index (LAI), is important for mapping the spatiotemporal variability of crop and soil conditions, and modeling crop nutrient balance, and crop productivity [
1,
2,
3]. LChl is the main light-harvesting pigment that determines leaf photosynthetic capacity, and it is highly influenced by nitrogen fertilization [
4,
5,
6]. Furthermore, incorporating LChl into process-based crop models could improve model performance [
7,
8]. LChl varies with leaf positions, species, crop types, crop growth stages and crop managements [
7,
9,
10]; thus, knowledge on the spatiotemporal variability of LChl is important to understand the status of crop growth condition and productivity [
8,
11,
12].
A number of studies have investigated the potential of remote sensing data in estimating LChl using statistical or physical based approaches [
13,
14,
15]. There has been rapid development in new satellite sensors, such as multispectral satellite sensors with red-edge (680–750 nm) reflectance measurements (e.g., Sentinel-2 and VENµS) [
16,
17], the VNIR-SWIR hyperspectral satellite sensors (e.g., HyspIRI and EnMAP) [
18,
19], and the multi- and hyperspectral imaging systems mounted on a UAV system [
19]. In particular, these sensors possess the red-edge or hyperspectral reflectance that is highly sensitive to changes in LChl [
4]. This allows for improved accuracy for LChl estimation from remote sensing data at different spatial scales. Accurate in situ LChl measurements are essential for developing and validating remote-sensing LChl estimation models.
Destructive and non-destructive methods are often used for LChl measurement. Both methods rely on measured light absorption/transmission to determine LChl [
2,
20,
21]. Conventionally, destructive measurement is conducted using a wet-chemical method in a lab setting [
4,
22]. Leaves are harvested from the plant and chlorophyll is extracted using organic solvents (e.g., acetone, methanol, ethanol, dimethyl sulphoxide (DMSO), or N-dimethyl formamide (DMF) [
4,
22,
23]. A spectrophotometer, a fluorometer, or a high-performance liquid chromatography (HPLC) is often used to measure light absorptions at a few wavelength ranges [
4,
23], which are then used to determine LChl. The lab-based approach is costly, labour intensive and time consuming. In addition, destructive sampling does not allow for tracking the temporal dynamics of LChl of the same leaves [
5].
Non-destructive methods provide a cost-efficient way for frequent measurement of LChl over a large area [
2,
9,
10]. Studies have found that spectral indices derived from light absorbance or reflectance at the visible and near infrared (NIR) regions have good correlations with LChl [
24,
25,
26,
27] and can be used to develop non-destructive methods for LChl measurements [
27,
28]. Portable chlorophyll meters (PCMs), such as the SPAD-502/501 (Soil Plant Analysis Development (SPAD) chlorophyll meter, Konica–Minolta, Inc., Osaka, Japan), the CCM-200 (CCM-200 plus Chlorophyll Content Meter, Opti-Sciences, Inc., Hudson, NH), and the Dualex-4 (Dualex Scientific
+TM Polyphenol & Chlorophyll Meter, FORCE-A, Orsay, France), have been developed for non-destructive measurements of LChl and nitrogen in the field [
2,
29,
30]. The readings from the PCMs (meter reading) are relative quantities that need to be converted to actual LChl. The transformation equations are usually established using meter readings and lab-measured LChl of the same leaf area [
21,
29,
30,
31,
32]. For instance, Markwell et al. [
21] developed a widely used exponential equation to estimate LChl from SPAD-502 readings, and Cerovic et al. [
29] subsequently developed a generic conversion function for SPAD-502 readings based on more data collected in different studies (e.g., Markwell et al. [
21], Richardson et al. [
30] and Marenco et al. [
33]).
It should be noted that factors other than LChl may also influence the light transmittance of a leaf, such as leaf structure, water content and leaf pigment distribution [
2,
34,
35]. Environmental factors such as light intensity can also affect light transmittance of a leaf, resulting in measurement errors of LChl [
36,
37]. Influences on light transmittance can be categorized into two groups [
38,
39,
40]. The first is the detour effect (light scattering), caused primarily by non-chlorophyll components (e.g., leaf architecture and dry matter), which can result in an increase in the path length of light inside a leaf [
41]. The sieve effect occurs when light passes through leaf tissues without being absorbed, thereby decreasing total absorption [
39,
40,
42]. The distribution of chlorophyll molecules within a leaf is usually non-uniform, associated with the structural organization of the grana within the chloroplasts, chloroplasts within the cells, and cells within the tissue layers [
43,
44]. Furthermore, the influences on light transmittance vary with wavelength. Since different PCMs are developed based on different wavelengths, they may be impacted differently by different factors. Large uncertainties have been reported when converting meter readings into LChl using a general conversion function for different crops [
29,
39]. An in depth understanding of the mechanisms for the PCMs is useful for improving protocols to obtain high-quality in situ LChl measurements. However, it is difficult to consider the impacts from all leaf/canopy and environmental factors through field experiments. Using a leaf radiative transfer model to simulate the complex light transmission processes inside a leaf may provide a solution [
14,
35,
43]. This study, therefore, was designed to address the following: (1) the performances of different PCMs in estimating actual LChl, (2) the relationships between PCM reading and the actual LChl, (3) the sources of errors in PCM measurements based on simulations of light transmission in a leaf using radiative transfer model, and (4) the potential of deriving a generic conversion equation for a specific PCM. To address these, an experiment was conducted to collect PCM readings of corn (
Zea mays L.), spring wheat (
Triticum aestivum L.)
, soybean
(Glycine max L. Merr.) and canola (
Brassica napus L.) using the three aforementioned instruments (SPAD-502, CCM-200 and Dualex-4) at the Central Experimental Farm (CEF) of Agriculture and Agri-Food Canada (AAFC) in Ottawa, during the 2016 growing season.
4. Discussion
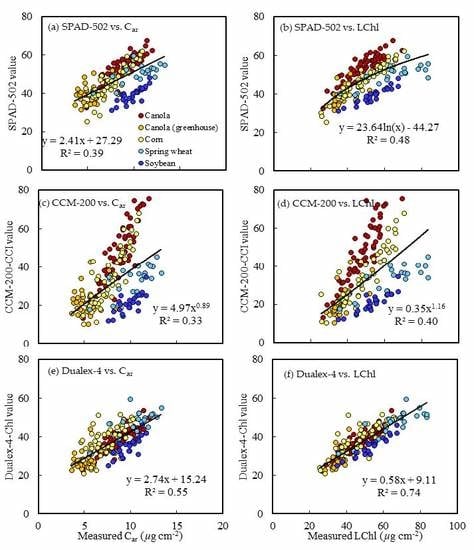
The four crops were selected to explore the capability of the three PCMs for crop LChl measurement. Leaf pigment contents were different among the four crops (
Figure 2 and
Table A1). In particular, the composition of LChl (i.e., Chl
a/Chl
b ratio,
Table A1) was obviously different between C4 plants (corn) and C3 plants (spring wheat, canola and soybean) because of their difference in photosynthesis pathways [
39,
56,
59,
60]. Actual LChl, especially high LChl, had a larger linear correlation coefficient with the Dualex-4-Chl readings than with the SPAD-502 and the CCM-200-CCI for an individual crop and for the four crops combined (
Table 4). Both SPAD-502 and CCM-200-CCI tend to have a nonlinear relationship with actual LChl, as they are sensitive to LChl at low LChl rates but are easily saturated at high LChl rates [
61]. These results are consistent with the results from Cerovic et al. [
29] and Casa et al. [
32], in which Dualex-4-Chl performed better than the other two instruments. More importantly, there is a greater potential to develop a generic calibration function for LChl estimates of the four crops using Dualex-4-Chl than using SPAD-502 and CCM-200-CCI. Dualex-4 could be more accurate and applicable than the other two PCMs in measuring LChl over a wide, dynamic range using a generic conversion function. Time and effort taken to recalibrate the concversion fuction for different crops would be largely reduced by using the Dualex-4 compared to the other two PCMs. Moreover, Dualex-4 has lower uncertainties at high LChl rates (
Figure 5 and
Figure 7). LChl generally shows an increasing trend during the vegetative stage, and reaches its maximum at the peak growing stage, which is an improtant inditor for asessing crop nitrogen uptake and crop yield [
7,
62]. Thus, Dualex-4 might be a better PCM to accuartely measure LChl at the peak growth stage.
Sensitivity analysis using the PROSPECT-D model simulation revealed that the leaf structure parameter (N
s) had a strong impact on the variability of PCM readings. The literature showed similar results; PCM readings were significantly affected by leaf internal architecture such as leaf thickness, specific leaf mass, and leaf succulence [
12,
27,
33,
58,
63]. Variability in the leaf structure parameter greatly influenced light interactions within the leaf, resulting in significant changes in light transmittance in the VIS and NIR ranges [
27,
35,
57]. Our study showed that the influence of leaf structure parameters was different on both the reference and the index bands. The influence of the parameters on the index band (710 nm) for Dualex-4-Chl was much greater than for SPAD-502 (650 nm) and CCM-200-CCI (653 nm), while the influence of the parameters on the reference band was comparable for all three PCMs (
Table 5). The influence of multiple scattering of light by leaf tissues could be further reduced by taking a simple ratio of NIR to VIS transmittance [
26,
27,
39,
58,
64]. The ratio of T850/T710 was better to restrict the influence of leaf structure parameters than T940/T650 and T937/T653. The logarithmic function applied to the ratio of 940/650 can restrict the influence of leaf structure parameters, especially at high LChl, and improve the sensitivity to C
ab. However, the overall ability of the three PCMs to reduce the impacts was limited (<15%), and their difference was small. The non-uniform distribution of LChl within the measured area was another important factor influencing the variation of PCM readings. The sensing area of CCM-200 (71 mm
2) is much larger than the sensing area of SPAD-502 (6 mm
2) and Dualex-4 (20 mm
2), hence CCM-200 can be more susceptible to greater non-uniformity in LChl distribution. In particular, CCM-200-CCI had larger variations at high LChl, compared with SPAD-200 and Dualex-4 (
Figure 4), which is consistent with the observations of Padilla et al. [
65]. Our simulation, using the method by Uddling et al. [
38], showed that CCM-200-CCI was most sensitive to the degree of non-uniformity of the LChl distribution, especially when LChl was high (
Figure 7). Increased heterogeneity of LChl distribution across leaf area can result in an increased light transmittance and decreased light absorption at the red wavelengths [
38]. Although similar wavelengths are employed in both SPAD-502 and CCM-200-CCI, the variability of SPAD-502 readings is not largely influenced by the heterogeneity distribution of LChl within the measured area. This could be attributed to the logarithmic transformation used in the SPAD-502, which helps reduce the divergence of non-uniform distribution of LChl [
39]. Dualex-4-Chl was the best at restricting the influence of the non-uniform LChl distribution. The data in this study showed that the meter readings for both SPAD-502 and CCM-200 had crop-specific relationships with LChl (
Figure 4). In particular, the deviations apparently increased at high rates of LChl. In addition, the errors of meter readings within the measured area increased with increasing LChl (
Figure 5). The results in both
Figure 4 and
Figure 5 are more consistent with
Figure 7 than with
Figure 6, suggesting that uncertainty in PCM readings was more due to non-uniform distribution of LChl than to leaf structure parameters. The studies of Parry et al. [
39] and Richardson et al. [
30] also showed that the non-uniform distribution of LChl at high LChl had an apparent influence on both SPAD-502 and CCM-200 readings. Previous studies [
20,
63] found that the heterogeneity of the pigment distribution was greater for leaves with a higher pigment content, as leaves with high chlorophyll concentration tend to have a high chlorophyll
density in chloroplasts, rather than develop more chloroplasts [
20].
The variations of light intensity can greatly influence chloroplast movement inside a leaf [
37,
40,
43,
66]. This can lead to variations of leaf optical properties (reflectance, absorbance and fluorescence) for the same amount of LChl, and therefore difference in PCM readings. Nauš et al. [
40] found that the movement of chloroplast from the cell walls perpendicular to the incident light (face position) to the cell walls parallel to the incident light (the side position) could induce approximately 35% of the difference in SPAD-502 readings. Padilla et al. [
36] reported that measurement time in day determines light intensity and can have a strong impact on SPAD-502 and CCM-200 readings. The ratio of Chl
a/Chl
b was considered to be an indicator of light intensity that has strong influence on chloroplast movement [
56,
67]. Strong relationships between the Chl
a/Chl
b ratio and SPAD-502 readings were observed by Netto et al. [
67] and Li et al. [
63]; however, we did not find similar relationships between the Chl
a/Chl
b ratio and PCM readings in the present study, similar to the result obtained by Parry et al. [
39]. The large difference in light conditions between the field and the greenhouse for canola was revealed by the Flav index of Dualex-4 measurements, which was another indicator of light intensity [
36,
47,
56]. In addition, the chloroplast movement is closely linked with the combined effects of light scattering (detour effect) and non-uniform chlorophyll distribution (sieve effect) [
40,
43,
66]. For instance, Nauš et al. [
40] reported that the impact of chloroplast movement on the relationship between SPAD readings and actual LChl was different between old and young tobacco leaves with different leaf structures (e.g., leaf area mass and leaf thickness). Our study results indirectly support this finding. In particular, the variability of the CCM-200-CCI readings was greatly affected by both light scattering and non-uniform LChl distribution. More recently, Stuckens et al. [
68] developed a Dorsiventral Leaf Model (DLM) to simulate leaf radiative transfer by considering the influence of leaf asymmetry that is modeled by assigning non-uniform distributions of pigments, water and dry matter to palisade and mesophyll layers and by simulating different amounts of light diffusion for adaxial and abaxial leaf surfaces. Baránková et al. [
43] developed a Simple Explicitly Non-Linear Empirical model for Leaf Optical Properties (SENLELOP model) to investigate the influence of chloroplast movement on the optical properties of green tobacco leaves. In future studies, a specially designed field experiment integrated with the SENLELOP model, DLM, or other, similar models, could lead to an improved understanding of the mechanistic relationship between optical readings and LChl.