Detecting the Sources of Methane Emission from Oil Shale Mining and Processing Using Airborne Hyperspectral Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. SASI Data and Preprocessing
2.3. Band Ratio for CH4 Emission Detection
- (1)
- Processing the spectrum with envelope removal can effectively highlight the absorption, reflection and emission characteristics of the spectral curve, and can normalize them to a consistent spectral background, which is conducive to the comparison of characteristic values with other spectral curves [32]. First is the envelope calculation of the spectral curve. Starting from the first point of the spectral curve, this is performed with the various points behind the attachment. The slope is calculated to determine the point of the maximum slope, which is the starting point of the next cycle. This is repeated until the last point of the spectrum curve. All slope maximum points are connected to form the envelope. Then, formula 4 is used to calculate the envelope-removed radiance (Figure 4).where RER is the envelope-removed radiance at the wavelength λ; Rλ is the radiance at the wavelength λ; and REλ is the envelope radiance at the wavelength λ.RER = Rλ/REλ
- (2)
- (3)
- The second layer high-frequency component is obtained after wavelet decomposition, and then the high-frequency component coefficient is calculated. The first layer of the high frequency component coming from the wavelet transform descomposition is the interference information for an extremely strong signal. The absorption features of CH4 are characterized by weak information. Weak information is easily lost after wavelet decomposition of the third layer. As such, the second layer of the high frequency component from wavelet decomposition will be used to characterize the detailed information of the spectral curve.
- (4)
- The singularity is detected to obtain the CH4 absorption bands, by calculating the modulus maximum of the second layer high-frequency component coefficient [30].
2.4. Extraction Method of the CH4 Release Source
3. Results
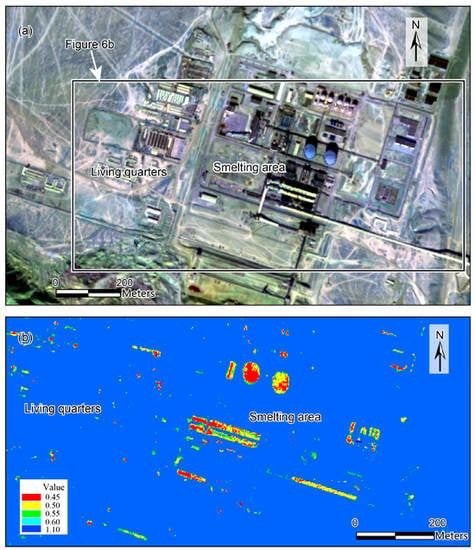
3.1. SASI Radiance Ratio Image for CH4 Emission Detection
3.2. CH4 Release Source Extracted from SASI Reflectance Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Saunois, M.; Jackson, R.B.; Bousquet, P.; Poulter, B.; Canadell, J.G. The growing role of methane in anthropogenic climate change. Environ. Res. Lett. 2016, 11, 120207. [Google Scholar] [CrossRef] [Green Version]
- Nisbet, E.G.; Dlugokencky, E.J.; Bousquet, P. Methane on the rise—Again. Science 2014, 343, 493–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anenberg, S.C.; Schwartz, J.; Shindell, D.; Amann, M.; Faluvegi, G.; Klimont, Z.; Janssens-Maenhout, G.; Pozzoli, L.; Van Dingenen, R.; Vignati, E.; et al. Global air quality and health co-benefits of mitigating near-term climate change through methane and black carbon emission controls. Environ. Health Perspect. 2012, 120, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelieveld, J.O.S.; Crutzen, P.J.; Dentener, F.J. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B 1998, 50, 128–150. [Google Scholar] [CrossRef]
- Hausmann, P.; Sussmann, R.; Smale, D. Contribution of oil and natural gas production to renewed increase of atmospheric methane (2007–2014): Top-down estimate from ethane and methane column observations. Atmos. Chem. Phys. 2016, 15, 35991–36028. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.; Sato, M.; Ruedy, R.; Lacis, A.; Oinas, V. Global warming in the twenty-first century: An alternative scenario. Proc. Acad. Natl. Sci. USA 2000, 18, 9875–9880. [Google Scholar] [CrossRef] [Green Version]
- Frankenberg, C.; Meirink, J.F.; van Weele, M.; Platt, U.; Wagner, T. Assessing methane emissions from global space-borne observations. Science 2005, 308, 1010–1014. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.A.; Bradley, E.S.; Cheung, R.; Leifer, I.; Dennison, P.E.; Margolis, J.S. Mapping methane emissions from a marine geological seep source using imaging spectrometry. Remote Sens. Environ. 2010, 114, 592–606. [Google Scholar] [CrossRef]
- Schneising, O.; Buchwitz, M.; Reuter, M.; Heymann, J.; Bovensmann, H.; Burrows, J.P. Long-term analysis of carbon dioxide and methane column-averaged mole fractions retrieved from SCIAMACH. Atmos. Chem. Phys. 2011, 11, 2863–2880. [Google Scholar] [CrossRef] [Green Version]
- Gerilowski, K.; Tretner, A.; Krings, T.; Buchwitz, M.; Bertagnolio, P.P.; Belemezov, F.; Erzinger, J.; Burrows, J.P.; Bovensmann, H. MAMAP—A new spectrometer system for column-averaged methane and carbon dioxide observations from aircraft: Instrument description and performance analysis. Atmos. Meas. Tech. 2011, 4, 215–243. [Google Scholar] [CrossRef] [Green Version]
- Krings, T.; Gerilowski, K.; Buchwitz, M.; Hartmann, J.; Sachs, T.; Erzinger, J.; Burrows, J.P.; Bovensmann, H. Quantification of methane emission rates from coal mine ventilation shafts using airborne remote sensing data. Atmos. Meas. Tech. 2013, 6, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, A.K.; Frankenberg, C.; Thompson, D.R.; Duren, R.M.; Aubrey, A.D.; Bue, B.D.; Green, R.O.; Gerilowski, K.; Krings, T.; Borchardt, J.; et al. Airborne DOAS retrievals of methane, carbon dioxide, and water vapor concentrations at high spatial resolution: Application to AVIRIS-NG. Atmos. Meas. Tech. 2017, 10, 3833–3850. [Google Scholar] [CrossRef] [Green Version]
- Scafutto, R.D.P.M.; De Souza Filho, C.R. Detection of Methane Plumes Using Airborne Midwave Infrared (3–5 µm) Hyperspectral Data. Remote Sens. 2018, 10, 1237. [Google Scholar] [CrossRef] [Green Version]
- Buchwitz, M.; Rozanov, V.V.; Burrows, J.P. A near-infrared optimized doas method for the fast global retrieval of atmospheric CH4, CO, CO2, H2O, and N2O total column amounts from sciamachy envisat-1 nadir radiances. J. Geophys. Res. Atmos. 2000, 105, 15231–15245. [Google Scholar] [CrossRef]
- Thompson, D.R.; Leifer, I.; Bovensmann, H.; Eastwood, M.; Fladeland, M.; Frankenberg, C. Real-time remote detection and measurement for airborne imaging spectroscopy: A case study with methane. Atmos. Meas. Tech. 2015, 8, 4383–4397. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, A.K.; Roberts, D.A.; Bradley, E.S.; Funk, C.C.; Dennison, P.E.; Leifer, I. High resolution mapping of methane emissions from marine and terrestrial sources using a Cluster-Tuned Matched Filter technique and imaging spectrometry. Remote Sens. Environ. 2013, 134, 305–318. [Google Scholar] [CrossRef]
- Larsen, N.F.; Stamnes, K. Methane detection from space: Use of sunglint. Opt. Eng. 2006, 45, 016202. [Google Scholar] [CrossRef]
- Bradley, E.S.; Leifer, I.; Roberts, D.A.; Dennison, P.E.; Washburn, L. Detection of marine methane emissions with AVIRIS band ratios. Geophys. Res. Lett. 2011, 10, 415–421. [Google Scholar] [CrossRef]
- Zhang, M.W.; Leifer, I.; Hu, C. Challenges in Methane Column Retrievals from AVIRIS-NG Imagery over Spectrally Cluttered Surfaces: A Sensitivity Analysis. Remote Sens. 2017, 9, 835. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.T.; Song, Z.G.; Luo, H.Y.; Liu, G.X. The difference of microscopic pore structure characteristics of coal, oil shale and shale and their storage mechanisms. Nat. Gas Geosic. 2015, 26, 2208–2218. [Google Scholar]
- Fuke, D.; Dong, Y.; Zijun, F. Permeability evolution of Jimsar oil shale under high temperature and triaxial stresses. Coal Technol. 2017, 36, 165–166. [Google Scholar]
- Campbell, J.H.; Gallegos, G.; Gregg, M. Gas evolution during oil shale pyrolysis. 2. Kinetic and stoichiometric analysis. Fuel 1980, 5910, 727–732. [Google Scholar] [CrossRef]
- Frankenberg, C.; Meirink, J.F.; Bergamaschi, P.; Goede, A.P.H.; Heimann, M.; Körner, S.; Platt, U.; Weele, M.V.; Wagner, T. Satellite cartography of atmospheric methane from SCIAMACHY on board ENVISAT: Analysis of the years 2003 and 2004. J. Geophys. Res. Atmos. 2006, 111, 7. [Google Scholar] [CrossRef]
- Straume, A.G.; Schrijver, H.; Gloudemans, A.M.S.; Houweling, S.; Aben, I.; Maurellis, A.N.; Laat, A.T.J.D.; Kleipool, Q.; Lichtenberg, G.; Hees, R.V. The global variation of CH4 and CO as seen by SCIAMACHY. Adv. Space Res. 2005, 36, 821–827. [Google Scholar] [CrossRef]
- Schepers, D.; Guerlet, S.; Butz, A.; Landgraf, J.; Frankenberg, C.; Hasekamp, O.; Blavier, J.F.; Deutscher, N.M.; Griffith, D.W.T.; Hase, F. Methane retrievals from Greenhouse Gases Observing Satellite (GOSAT) shortwave infrared measurements: Performance comparison of proxy and physics retrieval algorithms. J. Geophys. Res. Atmos. 2012, 117, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Kiehl, J.T.; Trenberth, K.E. Earth’s annual global mean energy budget. Bull. Am. Meteorol. Soc. 1997, 78, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Dennison, P.E.; Roberts, D.A. Daytime fire detection using airborne hyperspectral data. Remote Sens. Environ. 2009, 113, 1646–1657. [Google Scholar] [CrossRef]
- Lillesand, T.M.; Kiefer, R.W. Remote Sensing and Image Interpretation, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1994; pp. 7–8. ISBN 0471305758. [Google Scholar]
- Stephane, M.; Wen, L.H. Singularity detection and processing with wavelets. IEEE Trans. Inf. Theory 1992, 38, 617–634. [Google Scholar]
- Liu, Y.; Qin, F.; Liu, Y.; Cen, Z. The 2D large deformation analysis using Daubechies wavelet. Comput. Mech. 2010, 45, 179–187. [Google Scholar] [CrossRef]
- Huang, Z.; Turner, B.J.; Dury, S.J.; Wallis, I.R.; Foley, W.J. Estimating foliage nitrogen concentration from hymap data using continuum removal analysis. Remote Sens. Environ. 2004, 93, 18–29. [Google Scholar] [CrossRef]
- Xu, Y.; Weaver, J.B.; Healy, D.M.; Lu, J. Wavelet transform domain filters: A spatially selective noise filtration technique. IEEE Trans. Image Process. 1994, 3, 747–758. [Google Scholar] [PubMed] [Green Version]
- Zhang, X.D.; Younan, N.H.; O’Hara, C.G. Wavelet domain statistical hyperspectral soil texture classification. IEEE Trans. Geosci. Remote Sens. 2005, 43, 615–618. [Google Scholar] [CrossRef]
- Cho, M.A.; Debba, P.; Mathieu, R.; Naidoo, L.; van Aardt, J.; Asner, G.P. Improving discrimination of savanna tree species through a multiple-endmember spectral angle mapper approach: Canopy-level analysis. IEEE Trans. Geosci. Remote Sens. 2010, 48, 4133–4142. [Google Scholar] [CrossRef]
- Murphy, R.J.; Monteiro, S.T.; Schneider, S. Evaluating classification techniques for mapping vertical geology using field-based hyperspectral sensors. IEEE Trans. Geosci. Remote Sens. 2012, 50, 3066–3080. [Google Scholar] [CrossRef]
- Kruse, F.A.; Boardman, J.W.; Huntington, J.F. Comparison of Airborne Hyperspectral Data and EO-1 Hyperion for Mineral Mapping. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1388–1400. [Google Scholar] [CrossRef] [Green Version]
- Hecker, C.; Vander Meijde, M.; Vander Werff, H.; Vander Meer, F.D. Assessing the influence of reference spectra on synthetic SAM classification results. IEEE Trans. Geosci. Remote Sens. 2008, 46, 4162–4172. [Google Scholar] [CrossRef]
- Thorpe, A.K.; Frankenberg, C.; Roberts, D.A. Retrieval techniques for airborne imaging of methane concentrations using high spatial and moderate spectral resolution: Application to AVIRIS. Atmos. Meas. Tech. 2014, 7, 491–506. [Google Scholar] [CrossRef] [Green Version]
- Kruse, F.A.; Boardman, J.W.; Huntington, J.F. Comparison of airborne and satellite hyperspectral data for geologic mapping. Proc. SPIE Int. Soc. Opt. Eng. 2002, 4725, 128–139. [Google Scholar]










| Class | Mean Angle | Standard Deviation |
|---|---|---|
| 1 | 0.1 | 0.0082 |
| 2 | 0.1 | 0.0085 |
| 3 | 0.1 | 0.0086 |
| 4 | 0.1 | 0.0173 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; Fu, B.; Shui, H.; Guo, Z.; Zhu, J. Detecting the Sources of Methane Emission from Oil Shale Mining and Processing Using Airborne Hyperspectral Data. Remote Sens. 2020, 12, 537. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12030537
Xiao C, Fu B, Shui H, Guo Z, Zhu J. Detecting the Sources of Methane Emission from Oil Shale Mining and Processing Using Airborne Hyperspectral Data. Remote Sensing. 2020; 12(3):537. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12030537
Chicago/Turabian StyleXiao, Chunlei, Bihong Fu, Hanqing Shui, Zhaocheng Guo, and Jurui Zhu. 2020. "Detecting the Sources of Methane Emission from Oil Shale Mining and Processing Using Airborne Hyperspectral Data" Remote Sensing 12, no. 3: 537. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12030537