Broadacre Mapping of Wheat Biomass Using Ground-Based LiDAR Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experimental Design
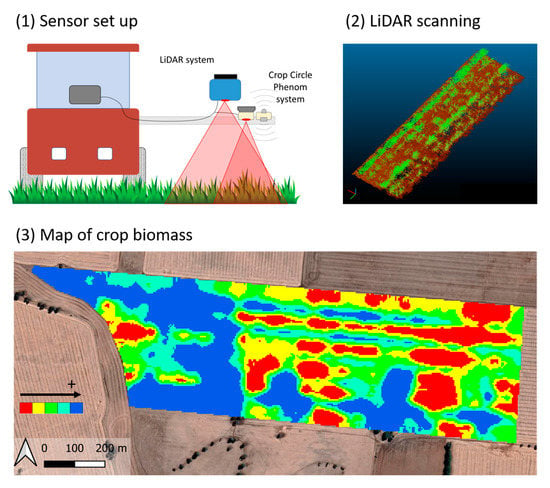
2.2. Sensor Setup
2.3. Data Processing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemaire, G.; Gastal, F. N Uptake and Distribution in Plant Canopies. In Diagnostics of the Nitrogen Status in Crops; Lemaire, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 3–43. [Google Scholar]
- Lemaire, G.; Sinclair, T.; Sadras, V.; Bélanger, G. Allometric approach to crop nutrition and implications for crop diagnosis and phenotyping. A review. Agron. Sustain. Dev. 2019, 39, 1–17. [Google Scholar]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage. Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Colaço, A.F.; Bramley, R.G.V. Do crop sensors promote improved nitrogen management in grain crops? Field Crop. Res. 2018, 218, 126–140. [Google Scholar] [CrossRef]
- Fitzgerald, G.J. Characterizing vegetation indices derived from active and passive sensors. Int. J. Remote Sens. 2010, 31, 4335–4348. [Google Scholar] [CrossRef]
- Rouse, J.W.; Hass, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite (ERTS) Symposium, Washington, DC, USA, 10–14 December 1973; Volume 351, p. 309. [Google Scholar]
- Tilling, A.K.; O’Leary, G.J.; Ferwerda, J.G.; Jones, S.D.; Fitzgerald, G.J.; Rodriguez, D.; Belford, R. Remote sensing of nitrogen and water stress in wheat. Field Crop. Res. 2007, 104, 77–85. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, H.-Y.; Zhang, Y.-S.; Qi, S.-L.; Heng, Y.-R.; Guo, B.-B.; Ma, D.-Y.; Guo, T.-C. Remote detection of canopy leaf nitrogen concentration in winter wheat by using water resistance vegetation indices from in-situ hyperspectral data. Field Crop. Res. 2016, 198, 238–246. [Google Scholar] [CrossRef]
- Franzen, D.W.; Kitchen, N.R.; Holland, K.H.; Schepers, J.S.; Raun, W.R. Algorithms for in-season nutrient management in cereals. Agron. J. 2016, 108, 1775. [Google Scholar] [CrossRef] [Green Version]
- Colaço, A.F.; Molin, J.P.; Rosell-Polo, J.R.; Escolà, A. Application of light detection and ranging and ultrasonic sensors to high-throughput phenotyping and precision horticulture: Current status and challenges. Hort. Res. 2018, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Escolà, A.; Rosell-Polo, J.R.; Planas, S.; Gil, E.; Pomar, J.; Camp, F.; Llorens, J.; Solanelles, F. Variable rate sprayer. Part 1—Orchard prototype: Design, implementation and validation. Comput. Electron. Agric. 2013, 95, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Colaço, A.F.; Trevisan, R.G.; Molin, J.P.; Rosell-Polo, J.R.; Escolà, A. A method to obtain orange crop geometry information using a mobile terrestrial laser scanner and 3d modeling. Remote Sens. 2017, 9, 763. [Google Scholar] [CrossRef] [Green Version]
- Colaço, A.F.; Molin, J.P.; Rosell-Polo, J.R.; Escolà, A. Spatial variability in commercial orange groves. Part 1: Canopy volume and height. Precis. Agric. 2019, 20, 788–804. [Google Scholar] [CrossRef] [Green Version]
- Siebers, M.H.; Edwards, E.J.; Jimenez-Berni, J.A.; Thomas, M.; Salim, M.; Walker, R. Fast phenomics in vineyards: Development of grover, the grapevine rover, and LiDAR for assessing grapevine traits in the field. Sensors 2018, 18, 2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworak, V.; Selbeck, J.; Ehlert, D. Ranging sensors for vehicle-based measurement of crop stand and orchard parameters: A review. Trans. ASABE 2011, 54, 1497–1510. [Google Scholar] [CrossRef]
- White, J.W.; Andrade-Sanchez, P.; Gore, M.A.; Bronson, K.F.; Coffelt, T.A.; Conley, M.M.; Feldmann, K.A.; French, A.N.; Heun, J.T.; Hunsaker, D.J.; et al. Field-based phenomics for plant genetics research. Field Crop. Res. 2012, 133, 101–112. [Google Scholar] [CrossRef]
- Lin, Y. LiDAR: An important tool for next-generation phenotyping technology of high potential for plant phenomics? Comput. Electron. Agric. 2015, 119, 61–73. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Bhatta, M.; Shi, Y.; Baenziger, P.S.; Ge, Y. Wheat height estimation using LiDAR in comparison to ultrasonic sensor and UAS. Sensors 2018, 18, 3731. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Berni, J.A.; Deery, D.M.; Rozas-Larraondo, P.; Condon, A.T.G.; Rebetzke, G.J.; James, R.A.; Bovill, W.D.; Furbank, R.T.; Sirault, X.R.R. High throughput determination of plant height, ground cover, and above-ground biomass in wheat with LiDAR. Front. Plant Sci. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.D.C.; Edwards, J.; McDonald, G.; Kuchel, H. Estimating biomass and canopy height with LiDAR for field crop breeding. Front. Plant Sci. 2019, 10, 1145. [Google Scholar] [CrossRef]
- Deery, D.M.; Rebetzke, G.J.; Jimenez-Berni, J.A.; Condon, A.G.; Smith, D.J.; Bechaz, K.M.; Bovill, W.D. Ground-based lidar improves phenotypic repeatability of above-ground biomass and crop growth rate in wheat. Plant Phenomics 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Deery, D.; Jimenez-Berni, J.; Jones, H.; Sirault, X.; Furbank, R. Proximal remote sensing buggies and potential applications for field-based phenotyping. Agronomy 2014, 4, 349–379. [Google Scholar] [CrossRef] [Green Version]
- Virlet, N.; Sabermanesh, K.; Sadeghi-Tehran, P.; Hawkesford, M.J. Field Scanalyzer: An automated robotic field phenotyping platform for detailed crop monitoring. Funct. Plant Biol. 2017, 44, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilly, N.; Hoffmeister, D.; Cao, Q.; Huang, S.; Lenz-Wiedemann, V.; Miao, Y.; Bareth, G. Multitemporal crop surface models: Accurate plant height measurement and biomass estimation with terrestrial laser scanning in paddy rice. J. Appl. Remote Sens. 2014, 8, 083671. [Google Scholar] [CrossRef]
- Ehlert, D.; Adamek, R.; Horn, H.J. Laser rangefinder-based measuring of crop biomass under field conditions. Precis. Agric. 2009, 10, 395–408. [Google Scholar] [CrossRef]
- Ehlert, D.; Schmerler, J.; Voelker, U. Variable rate nitrogen fertilisation of winter wheat based on a crop density sensor. Precis. Agric. 2004, 5, 263–273. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Magney, T.S.; Vierling, L.A.; Brown, T.T.; Huggins, D.R. LiDAR based biomass and crop nitrogen estimates for rapid, non-destructive assessment of wheat nitrogen status. Field Crop. Res. 2014, 159, 21–32. [Google Scholar] [CrossRef]
- Shendryk, Y.; Sofonia, J.; Garrard, R.; Rist, Y.; Skocaj, D.; Thorburn, P. Fine-scale prediction of biomass and leaf nitrogen content in sugarcane using UAV LiDAR and multispectral imaging. Int. J. Appl. Earth Obs. 2020, 92, 102177. [Google Scholar] [CrossRef]
- Bates, J.S.; Montzka, C.; Schmidt, M.; Jonard, F. Estimating canopy density parameters time-series for winter wheat using UAS mounted lidar. Remote Sens. 2021, 13, 710. [Google Scholar] [CrossRef]
- Long, D.S.; McCallum, J.D. Mapping straw yield using on-combine light detection and ranging (lidar). Int. J. Remote Sens. 2013, 34, 6121–6134. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Magney, T.S.; Vierling, L.A.; Greaves, H.E.; Zheng, G. An automated method to quantify crop height and calibrate satellite-derived biomass using hypertemporal lidar. Remote Sens. Environ. 2016, 187, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Colaço, A.F.; Bramley, R.G.V. A Spatially Distributed On-Farm Experimental Approach for the Development of a Sensor-Based Nitrogen Decision Model. In Proceedings of the 19th Australian Agronomy Conference, Wagga Wagga, NSW, Australia, 25–29 August 2019; The Australian Society of Agronomy: Toowoomba, Australia; pp. 1–4. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal growth code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Stanford Artificial Intelligence Laboratory; Robotic Operating System. 2018. Available online: https://www.ros.org (accessed on 8 October 2020).
- Holland, K.H.; Lamb, D.W.; Schepers, J.S. Radiometry of proximal active optical sensors (AOS) for agricultural sensing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 1793–1802. [Google Scholar] [CrossRef]
- Cloud Compare v2.10.2 [GPL Software]. 2020. Available online: http://www.cloudcompare.org (accessed on 8 October 2020).
- Zhang, W.; Qi, J.; Wan, P.; Wang, H.; Xie, D.; Wang, X.; Yan, G. An Easy-to-Use Airborne LiDAR Data Filtering Method Based on Cloth Simulation. Remote Sens. 2016, 8, 501. [Google Scholar] [CrossRef]
- QGIS v3.10—QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. Available online: http://www.qgis.org. (accessed on 8 October 2020).
- Ratcliff, C.; Gobbett, D.; Bramley, R.G.V. PAT—Precision Agriculture Tools. v3. CSIRO. Software Collections. 2020. Available online: https://0-doi-org.brum.beds.ac.uk/10.25919/5f72d61b0bca9 (accessed on 30 June 2021). [CrossRef]
- Minasny, B.; McBratney, A.B.; Whelan, B.M. VESPER Version 1.62. Australian Centre for Precision Agriculture, McMillan Building A05, the University of Sydney, NSW. 2005. Available online: https://precision-agriculture.sydney.edu.au/resources/software/download-vesper (accessed on 8 October 2020).
- Underwood, J.; Wendel, A.; Schofield, B.; McMurray, L.; Kimber, R. Efficient in-field plant phenomics for row-crops with an autonomous ground vehicle. J. Field Robot. 2017, 34, 1061–1083. [Google Scholar] [CrossRef]
- Hammerle, M.; Hofle, B. Effects of Reduced Terrestrial LiDAR Point Density on High-Resolution Grain Crop Surface Models in Precision Agriculture. Sensors 2014, 14, 24212–24230. [Google Scholar] [CrossRef] [Green Version]
- Gebbers, R.; Ehlert, D.; Adamek, R. Rapid mapping of the leaf area index in agricultural crops. Agron. J. 2011, 103, 1532. [Google Scholar] [CrossRef]
- Ehlert, D.; Heisig, M. Sources of angle-dependent errors in terrestrial laser scanner-based crop stand measurement. Comput. Electron. Agr. 2013, 93, 10–16. [Google Scholar] [CrossRef]
- Méndez, V.; Catalán, H.; Rosell, J.R.; Arnó, J.; Sanz, R.; Tarquis, A. SIMLIDAR—Simulation of LIDAR performance in artificially simulated orchards. Biosyst. Eng. 2012, 111, 72–82. [Google Scholar] [CrossRef] [Green Version]









| Treatment | MAP a (15/05/19) Sowing | UAN b (11 June 2019) Two-Leaf Stage | Urea (27 June 2019) Tillering | Urea (2 August 2019) Stem Elongation | Total |
|---|---|---|---|---|---|
| N (kg ha−1) | |||||
| N ‘minus’ | 11.1 | - | - | 42.4 * | 53.5 |
| Field | 11.1 | - | 46 | 35.1 * | 92.2 |
| N ‘rich’ | 11.1 | 84.5 | 46 | 36.5 * | 178.1 |
| N% | DW | Hm | NDRE | NDVI | PAR | Hcc | COVL | HL | |
|---|---|---|---|---|---|---|---|---|---|
| N% | 1.00 | ||||||||
| DW | 0.02 | 1.00 | |||||||
| Hm | 0.04 | 0.56 | 1.00 | ||||||
| NDRE | 0.49 | 0.45 | 0.36 | 1.00 | |||||
| NDVI | 0.48 | 0.37 | 0.37 | 0.97 | 1.00 | ||||
| PAR | 0.07 | 0.45 | 0.51 | 0.58 | 0.60 | 1.00 | |||
| Hcc | 0.38 | 0.01 | 0.03 | 0.26 | 0.28 | 0.03 | 1.00 | ||
| COVL | 0.42 | 0.47 | 0.54 | 0.85 | 0.86 | 0.58 | 0.23 | 1.00 | |
| HL | 0.10 | 0.63 | 0.63 | 0.55 | 0.53 | 0.60 | 0.03 | 0.77 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colaço, A.F.; Schaefer, M.; Bramley, R.G.V. Broadacre Mapping of Wheat Biomass Using Ground-Based LiDAR Technology. Remote Sens. 2021, 13, 3218. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13163218
Colaço AF, Schaefer M, Bramley RGV. Broadacre Mapping of Wheat Biomass Using Ground-Based LiDAR Technology. Remote Sensing. 2021; 13(16):3218. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13163218
Chicago/Turabian StyleColaço, André Freitas, Michael Schaefer, and Robert G. V. Bramley. 2021. "Broadacre Mapping of Wheat Biomass Using Ground-Based LiDAR Technology" Remote Sensing 13, no. 16: 3218. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13163218