Trans-Cinnamic Acid Stimulates White Fat Browning and Activates Brown Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Differentiation
2.3. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.4. Oil Red O Staining
2.5. Immunoblot Analysis
2.6. Immunocytochemistry
2.7. Statistical Analysis
3. Results
3.1. Trans-Cinnamic Acid (tCA) Induces Browning in 3T3-L1 White Adipocytes
3.2. tCA Activates HIB1B Brown Adipocytes
3.3. tCA Promotes Mitochondrial Biogenesis in White and Brown Adipocytes
3.4. tCA Regulates Lipid Metabolism in White Adipocytes
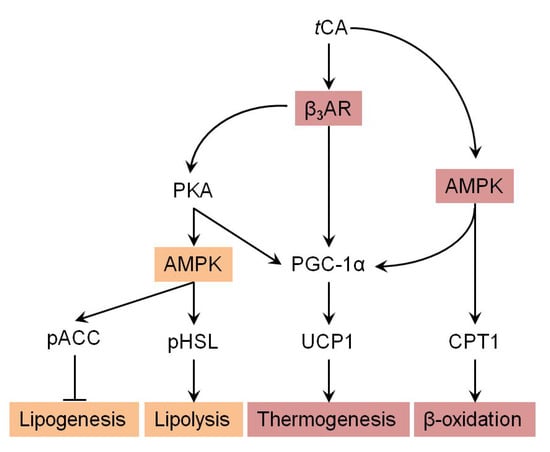
3.5. tCA Induces Browning of White Adipocytes via Activation of the β3-AR and AMPK Signaling Pathways
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | acyl-CoA carboxylase |
| ACO | acyl-coenzyme A oxidase 1 |
| AMPK | AMP-activated protein kinase |
| AR | adrenergic receptor |
| ATGL | adipose triglyceride lipase |
| BAT | brown adipose tissue |
| Beige | brown in white |
| Cd137 | tumor necrosis factor receptor superfamily, member 9 |
| Cidea | gene encoding cell death-inducing DFFA-like effector a |
| Cited1 | gene encoding Cbp/p300-interacting transactivator 1 |
| C/EBP/Cebp | CCAAT/enhancer-binding protein/encoding gene |
| Cox4 | cytochrome c oxidase subunit 4 |
| CPT1 | carnitine palmitoyltransferase 1 |
| FAS | fatty acid synthase |
| HSL | hormone-sensitive lipase |
| Lhx8 | gene encoding LIM/homeobox protein Lhx8 |
| NRF1 | nuclear respiratory factor 1 |
| PGC-1α/Ppargc1α | peroxisome proliferator-activated receptor gamma co-activator 1-alpha/encoding gene |
| PKA | protein kinase A |
| PPAR | peroxisome proliferator-activated receptor |
| PRDM16/Prdm16 | PR domain-containing 16/encoding gene |
| tCA | trans-cinnamic acid |
| Tbx1 | gene encoding T-box protein 1 |
| Tfam | transcription factor A, mitochondrial |
| Tmem26 | gene encoding transmembrane protein 26 |
| UCP1/Ucp1 | uncoupling protein 1/encoding gene |
| Zic1 | gene encoding zinc finger protein ZIC1 |
References
- Mnafgui, K.; Derbali, A.; Sayadi, S.; Gharsallah, N.; Elfeki, A.; Allouche, N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet-induced obese rats. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes (Lond.) 2007, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.Y.; Ishikawa, K.; Virtue, S.; Vidal-Puig, A. Brown adipose tissue in the treatment of obesity and diabetes: Are we hot enough? J. Diabetes Investig. 2011, 2, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, A.L.; Vorlander, C.; Reddy-Luthmoodoo, M.; Natoli, A.K.; Formosa, M.F.; Bertovic, D.A.; Anderson, M.J.; Duffy, S.J.; Kingwell, B.A. Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PLoS ONE 2014, 9, e91997. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kang, N.H.; Mukherjee, S.; Yun, J.W. Theobromine, a methylzanthine in cocoa bean, stimulates thermogenesis by inducing white fat browning and activating brown adipocytes. Biotechnol. Bioprocess Eng. 2018, 23, 617–626. [Google Scholar] [CrossRef]
- Claussnitzer, M.; Dankel, S.N.; Kim, K.H.; Quon, G.; Meuleman, W.; Haugen, C.; Glunk, V.; Sousa, I.S.; Beaudry, J.L.; Puviindran, V.; et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 2015, 373, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, P. Wasting energy to treat obesity. N. Engl. J. Med. 2016, 375, 2298–2300. [Google Scholar] [CrossRef]
- Sellayah, D.; Sikder, D. Orexin restores aging-related brown adipose tissue dysfunction in male mice. Endocrinology 2014, 155, 485–501. [Google Scholar] [CrossRef]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Ricquier, D. Uncoupling protein 1 of brown adipocytes, the only uncoupler: A historical perspective. Front. Endocrinol. (Lausanne) 2011, 2, 85. [Google Scholar] [CrossRef]
- Azzu, V.; Brand, M.D. The on-off switches of the mitochondrial uncoupling proteins. Trends. Biochem. Sci. 2010, 35, 298–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parray, H.A.; Yun, J.W. Combined inhibition of autophagy protein 5 and galectin-1 by thiodigalactoside reduces diet-induced obesity through induction of white fat browning. IUBMB Life 2017, 69, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Tiraby, C.; Tavernier, G.; Lefort, C.; Larrouy, D.; Bouillaud, F.; Ricquier, D.; Langin, D. Acquirement of brown fat cell features by human white adipocytes. J. Biol. Chem. 2003, 278, 33370–33376. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V.; et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Werner, C.D.; Kebebew, E.; Celi, F.S. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int. J. Obes. (Lond.) 2014, 38, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Ciniti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef]
- Barquissau, V.; Beuzelin, D.; Pisani, D.F.; Beranger, G.E.; Mairal, A.; Montagner, A.; Roussel, B.; Tavrnier, G.; Marques, M.A.; Moro, C.; et al. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol. Metab. 2016, 5, 352–365. [Google Scholar] [CrossRef]
- Bonet, M.L.; Oliver, P.; Palou, A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta. 2018, 1831, 969–985. [Google Scholar] [CrossRef]
- Azhar, Y.; Parmar, A.; Miller, C.N.; Samuels, J.S.; Rayalam, S. Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr. Metab. (Lond.) 2016, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.H.; Mukherjee, S.; Min, T.; Kang, S.C.; Yun, J.W. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochimie 2018, 151, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Yun, J.W. Honokiol exerts dual effects on browning and apoptosis of adipocytes. Pharmacol. Rep. 2017, 69, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Cinnamon (Cinnamomum Species) Medical Toxicology of Natural Substances; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 327–335. [Google Scholar]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based. Complement. Alternat. Med. 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Scgoene, N.W.; Graves, D.F. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J. Agric. Food. Chem. 2014, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Attia, H.F.; El-Shazly, A.; Saleh, O.M. Biomedical effects of cinnamon extract on obesity and diabetes relevance in wistar rats. AJMB 2012, 3, 133–145. [Google Scholar] [CrossRef]
- Jain, G.S.; Puri, S.; Misra, A.; Gulati, S.; Mani, K. Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: A randomized double -blind control trial. Lipids Health Dis. 2017, 16, 113. [Google Scholar] [CrossRef]
- Kopp, C.; Singh, S.P.; Regenhard, P.; Müller, U.; Sauerwein, H.; Mielenz, M. Trans-cinnamic acid increases adiponectin and the phosphorylation of AMP-activated protein kinase through G-protein-coupled receptor signaling in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2014, 15, 2906–2915. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Method 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Qin, B.; Panickar, K.S.; Anderson, A.R. Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J. Diabetes. Sci. Technol. 2010, 4, 685–693. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, H.; Ververis, K.; Karagiannis, T.C. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes. Obes. Metab. 2012, 14, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Verspohl, E.J.; Bauer, K.; Neddermann, E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother. Res. 2005, 1, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Camacho, S.; Michlig, S.; de Senarclens-Bezençon, C.; Meylan, J.; Meystre, J.; Pezzoli, M.; Markram, H.; le Coutre, J. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci. Rep. 2015, 5, 7919. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Wu, J.; Su, T.; Chao, X.J.; Liu, B.; Fu, X.; Chan, C.L.; Lau, R.H.Y.; Tse, A.K.W.; Han, Q.B.; et al. Cinnamon induces browning in subcutaneous adipocytes. Sci. Rep. 2015, 7, 2447. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Iwasaki, Y.; Narukawa, M.; Watanabe, T. Ingestion of cinnamaldehyde, a TRPA1 agonist, reduces visceral fats in mice fed a high-fat and high-sucrose diet. J. Nutr. Sci. Vitaminol (Tokyo) 2012, 58, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tang, Z. Expression and regulation of the ERK1/2 and p38 MAPK signaling pathways in periodontal tissue remodeling of orthodontic tooth movement. Mol. Med. Rep. 2018, 17, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes. Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.L.; Yen, G.C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Baile, C.A.; Zhu, S.; Shoji, M. Bioactive flavonoid p-hydroxycinnamic acid stimulates osteoblastogenesis and suppresses adipogenesis in bone marrow culture. Cell Tissue Res. 2013, 354, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Liu, C.; Wang, A.; Sun, Q. Intermittent cold exposure improves glucose homeostasis associated with brown and white adipose tissues in mice. Life Sci. 2015, 15, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.; Kim, H.; Noratto, G.; Sun, Y.; Talcott, S.T.; Mertens-Talcott, S.U. Gallotannin derivatives from mango (Mangifera indica L.) suppress adipogenesis and increase thermogenesis in 3T3-L1 adipocytes in part through the AMPK pathway. J. Funct. Foods 2018, 46, 101–109. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Interaction of cinnamic acid derivatives with commercial hypoglycemic drugs on 2-deoxyglucose uptake in 3T3-L1 adipocytes. J. Agric. Food Chem. 2011, 5, 9835–9844. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, E.P.; Balasubramanian, P.; Lee, Y.H.; Weng, C.; Kershaw, E.E.; Granneman, J.G. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J. Lipid Res. 2014, 55, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Dong, L.; Liu, F. Recent advances in adipose mTOR signaling and function: Therapeutic prospects. Trends Pharmacol. Sci. 2016, 37, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, Y.; Wang, C.; Burge, M.; Yang, X.O.; Liu, M. Adipose mTORC1 suppresses prostaglandin signaling and beige adipogenesis via the CRTC2-COX-2 pathway. Cell Rep. 2018, 24, 3180–3193. [Google Scholar] [CrossRef]
- Yin, W.; Mu, J.; Birnbaum, M.J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 2003, 278, 43074–43080. [Google Scholar] [CrossRef]
- Daval, M.; Diot-Dupuy, F.; Bazin, R.; Hainault, I.; Viollet, B.; Vaulont, S.; Hajduch, E.; Ferré, P.; Foufelle, F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 2005, 280, 25250–25257. [Google Scholar] [CrossRef]
- Kalinovich, A.V.; de Jong, J.M.; Cannon, B.; Nedergaard, J. UCP1 in adipose tissues: Two steps to full browning. Biochimie 2017, 134, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Yun, J.W. Monoterpene limonene induces brown fat-like phenotype in 3T3-L1 white adipocytes. Life Sci. 2016, 15, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Parray, H.A.; Yun, J.W. Cannabidiol promotes browning in 3T3L1 adipocytes. Mol. Cell Biochem. 2016, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xie, Y.; Yang, Q.; Cao, Y.; Tu, H.; Cao, W.; Wang, S. Pharmacokinetic study of cinnamaldehyde in rats by GC-MS after oral and intravenous administration. J. Pharm. Biomed. Anal. 2014, 89, 150–157. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Nutley, B.P.; Farmer, P.; Caldwell, J. Metabolism of trans-cinnamic acid in the rat and the mouse and its variation with dose. Food Chem. Toxic. 1994, 32, 877–886. [Google Scholar] [CrossRef]






| Gene | Accession No. | Forward | Reverse |
|---|---|---|---|
| Cd137 | DQ832278.1 | GGTCTGTGCTTAAGACCGGG | TCTTAATAGCTGGTCCTCCCTC |
| Cidea | NM_007702.2 | CGGGAATAGCCAGAGTCACC | TGTGCATCGGATGTCGTAGG |
| Cited1 | NM_001276466.1 | GGAAGGCACAGCACCCACTC | GGAAGGCACAGCACCCACTC |
| Cox4 | NM_001293559.1 | TGACGGCCTTGGACGG | CGATCAGCGTAAGTGGGGA |
| Lhx8 | NM_010713.2 | CATCGCTGTTCTGCCTGTTAG | CTCGGGATTCAGCAGTCCTTC |
| Nrf1 | NM_010938.4 | GCTAATGGCCTGGTCCAGAT | CTGCGCTGTCCGATATCCTG |
| Ppargc1α | NM_008904.2 | ATGAATGCAGCGGTCTTAGC | AACAATGGCAGGGTTTGTTC |
| Prdm16 | NM_027504.3 | GATGGGAGATGCTGACGGAT | TGATCTGACACATGGCGAGG |
| Tbx1 | NM_001285472.1 | AGCGAGGCGGAAGGGA | CCTGGTGACTGTGCTGAAGT |
| Tfam | BC083084.1 | ATGTGGAGCGTGCTAAAAGC | GGATAGCTACCCATGCTGCTGGAA |
| Tmem26 | NM_177794.3 | CCATGGAAACCAGTATTGCAGC | ATTGGTGGCTCTGTGGGATG |
| Ucp1 | NM_009463.3 | CCTGCCTCTCTCGGAAACAA | GTAGCGGGGTTTGATCCCAT |
| Zic1 | NM_009573.3 | GCCACAAATCCGGGAAGAAG | CTCACTTTCTCGCCGCTCAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.H.; Mukherjee, S.; Yun, J.W. Trans-Cinnamic Acid Stimulates White Fat Browning and Activates Brown Adipocytes. Nutrients 2019, 11, 577. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11030577
Kang NH, Mukherjee S, Yun JW. Trans-Cinnamic Acid Stimulates White Fat Browning and Activates Brown Adipocytes. Nutrients. 2019; 11(3):577. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11030577
Chicago/Turabian StyleKang, Nam Hyeon, Sulagna Mukherjee, and Jong Won Yun. 2019. "Trans-Cinnamic Acid Stimulates White Fat Browning and Activates Brown Adipocytes" Nutrients 11, no. 3: 577. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11030577