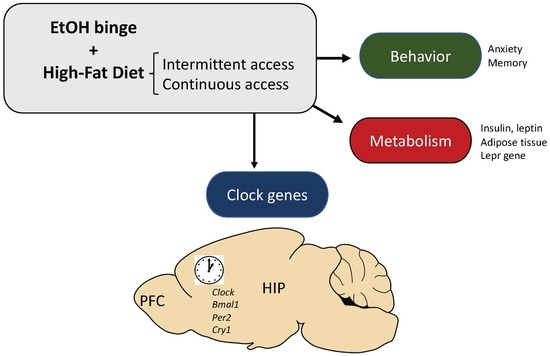
Differential Impact of Ad Libitum or Intermittent High-Fat Diets on Bingeing Ethanol-Mediated Behaviors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Procedure
2.3.1. Feeding Conditions
2.3.2. Drug Pre-Exposure Procedure
2.3.3. Experimental Design
2.4. Apparatus
2.4.1. Elevated Plus Maze
2.4.2. Object Recognition
2.4.3. Passive Avoidance Test
2.5. Plasma Biochemistry
2.6. Quantitative Real-Time PCR
2.7. Statistics
3. Results
3.1. Ad Libitum HFD, but Not Binge HFD, Increased Body Weight
3.2. Binge HFD Increased Adiposity Levels and Plasma Leptin Only in Mice that Consumed Ethanol
3.3. Mice that Binge on HFD Showed an Anxiolytic Profile
3.4. HFD Counteracts the Impairment in the Object Recognition Test Induced by EtOH
3.5. EtOH Affected Memory in the Passive Avoidance Test
3.6. Ad Libitum HFD Increased Lepr Gene Expression within the Hippocampus and the Prefrontal Cortex
3.7. Ad Libitum HFD Altered Clock Genes Expression within the Hippocampus and the Prefrontal Cortex
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz, J.A. Dietary habits and nutritional status in adolescents over Europe—Southern Europe. Eur. J. Clin. Nutr. 2000, 54 (Suppl. 1), S29. [Google Scholar] [CrossRef]
- Schneider, M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict. Boil. 2008, 13, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Bava, S.; Tapert, S.F. Adolescent Brain Development and the Risk for Alcohol and Other Drug Problems. Neuropsychol. Rev. 2010, 20, 398–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baladi, M.G.; Daws, L.C.; France, C.P. You are what you eat: Influence of type and amount of food consumed on central dopamine systems and the behavioral effects of direct-and indirect-acting dopamine receptor agonists. Neuropharmacology 2012, 63, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B. Adolescent eating disorders: Update on definitions, symptomatology, epidemiology, and comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Ifland, J.; Preuss, H.; Marcus, M.; Rourke, K.; Taylor, W.; Burau, K.; Jacobs, W.; Kadish, W.; Manso, G. Refined food addiction: A classic substance use disorder. Med. Hypotheses 2009, 72, 518–526. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef]
- Gold, M.S. From bedside to bench and back again: A 30-year saga. Physiol. Behav. 2011, 104, 157–161. [Google Scholar] [CrossRef]
- Heyward, F.D.; Walton, R.G.; Carle, M.S.; Coleman, M.A.; Garvey, W.T.; Sweatt, J.D. Adult Mice Maintained on a High-Fat Diet Exhibit Object Location Memory Deficits and Reduced Hippocampal SIRT1 Gene Expression. Neurobiol. Learn. Mem. 2012, 98, 25–32. [Google Scholar] [CrossRef]
- McNay, E.C.; Ong, C.T.; McCrimmon, R.J.; Cresswell, J.; Bogan, J.S.; Sherwin, R.S. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010, 93, 546–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Morales, L.; Ruiz-Gayo, M.; Del Olmo, N. Effect of high-fat diets on mood and learning performance in adolescent mice. Behav. Brain Res. 2016, 311, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Valladolid-Acebes, I.; Fole, A.; Martin, M.; Morales, L.; Cano, M.V.; Ruiz-Gayo, M.; Del Olmo, N. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol. Learn. Mem. 2013, 106, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Valladolid-Acebes, I.; Stucchi, P.; Cano, V.; Fernandez-Alfonso, M.S.; Merino, B.; Gil-Ortega, M.; Fole, A.; Morales, L.; Ruiz-Gayo, M.; Del Olmo, N. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol. Learn. Mem. 2011, 95, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Del Olmo, N.; Valladolid-Acebes, I.; Fole, A.; Cano, V.; Merino, B.; Stucchi, P.; Ruggieri, D.; López, L.; Alguacil, L.F.; et al. Shift of Circadian Feeding Pattern by High-Fat Diets Is Coincident with Reward Deficits in Obese Mice. PLoS ONE 2012, 7, e36139. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gandía, M.C.; Ledesma, J.C.; Aracil-Fernández, A.; Navarrete, F.; Montagud-Romero, S.; Aguilar, M.A.; Manzanares, J.; Miñarro, J.; Rodríguez-Arias, M. The rewarding effects of ethanol are modulated by binge eating of a high-fat diet during adolescence. Neuropharmacology 2017, 121, 219–230. [Google Scholar] [CrossRef]
- Blanco-Gandía, M.C.; Cantacorps, L.; Aracil-Fernández, A.; Montagud-Romero, S.; Aguilar, M.A.; Manzanares, J.; Valverde, O.; Miñarro, J.; Rodríguez-Arias, M. Effects of bingeing on fat during adolescence on the reinforcing effects of cocaine in adult male mice. Neuropharmacology 2017, 113, 31–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Gandía, M.C.; Aracil-Fernández, A.; Montagud-Romero, S.; Aguilar, M.A.; Manzanares, J.; Miñarro, J.; Rodríguez-Arias, M. Changes in gene expression and sensitivity of cocaine reward produced by a continuous fat diet. Psychopharmacology 2017, 192, 500–2352. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gandía, M.C.; Miñarro, J.; Rodríguez-Arias, M. Behavioral profile of intermittent vs continuous access to a high fat diet during adolescence. Behav. Brain Res. 2019, 368, 111891. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Walter, L.; Ricketts, H.; Whitlock, G.; Law, B.; Norton, R.; Fletcher Challenge-University of Auckland Heart and Health Study Management Committee. The determinants of fat intake in a multi-ethnic New Zealand population. Int. J. Epidemiol. 1998, 27, 416–421. [Google Scholar] [CrossRef]
- Stickley, A.; Koyanagi, A.; Koposov, R.; McKee, M.; Murphy, A.; Ruchkin, V. Binge Drinking and Eating Problems in Russian Adolescents. Alcohol. Clin. Exp. Res. 2015, 39, 540–547. [Google Scholar] [CrossRef]
- Mann, A.P.; Accurso, E.C.; Stiles-Shields, C.; Capra, L.; Labuschagne, Z.; Karnik, N.S.; Le Grange, D. Factors Associated with Substance Use in Adolescents with Eating Disorders. J. Adolesc. Heal. 2014, 55, 182–187. [Google Scholar] [CrossRef]
- Sturman, D.A.; Moghaddam, B. Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. J. Neurosci. 2011, 31, 1471–1478. [Google Scholar] [CrossRef]
- Management of Substance Abuse Unit. Global Status Report on Alcohol and Health, 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Carbia, C.; Cadaveira, F.; Caamaño-Isorna, F.; Rodríguez-Holguín, S.; Corral, M. Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS ONE 2017, 12, e0171393. [Google Scholar] [CrossRef]
- Jang, J.B.; Patrick, M.E.; Keyes, K.M.; Hamilton, A.D.; Schulenberg, J.E. Frequent binge drinking among US adolescents, 1991 to 2015. Pediatrics 2017, 139, e20164023. [Google Scholar] [CrossRef] [PubMed]
- Guerri, C. Mechanisms involved in central nervous system dysfunctions induced by prenatal ethanol exposure. Neurotox. Res. 2002, 4, 327–335. [Google Scholar] [CrossRef]
- Guerri, C.; Pascual, M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 2010, 44, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Blanco, A.M.; Cauli, O.; Miñarro, J.; Guerri, C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 2007, 25, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Varlinskaya, E.I.; Kim, E.U.; Spear, L.P. Chronic intermittent ethanol exposure during adolescence: Effects on stress-induced social alterations and social drinking in adulthood. Brain Res. 2017, 1654, 145–156. [Google Scholar] [CrossRef]
- Brown, S.A.; McGue, M.; Maggs, J.; Schulenberg, J.; Hingson, R.; Swartzwelder, S.; Martin, C.; Chung, T.; Tapert, S.F.; Sher, K.; et al. A Developmental Perspective on Alcohol and Youths 16 to 20 Years of Age. Pediatrics 2008, 121 (Suppl. 4), S290–S310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrallah, N.A.; Yang, T.W.H.; Bernstein, I.L. Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proc. Natl. Acad. Sci. USA 2009, 106, 17600–17604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peleg-Raibstein, D.; Sarker, G.; Litwan, K.; Krämer, S.D.; Ametamey, S.M.; Schibli, R.; Wolfrum, C. Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Transl. Psychiatry 2016, 6, e911. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Romero, S.; Daza-Losada, M.; Vidal-Infer, A.; Maldonado, C.; Aguilar, M.A.; Miñarro, J.; Rodríguez-Arias, M. The Novelty-Seeking Phenotype Modulates the Long-Lasting Effects of Intermittent Ethanol Administration during Adolescence. PLoS ONE 2014, 9, e92576. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, J.C.; Aguilar, M.A.; Gíménez-Gómez, P.; Miñarro, J.; Rodríguez-Arias, M. Adolescent but not adult ethanol binge drinking modulates cocaine withdrawal symptoms in mice. PLoS ONE 2017, 12, e0172956. [Google Scholar] [CrossRef] [PubMed]
- Manev, H.; Uz, T. Clock genes: Influencing and being influenced by psychoactive drugs. Trends Pharmacol. Sci. 2006, 27, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Perreau-Lenz, S.; Spanagel, R. Clock genes × stress × reward interactions in alcohol and substance use disorders. Alcohol 2015, 49, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Corwin, R.L.; Wojnicki, F.H.; Fisher, J.O.; Dimitriou, S.G.; Rice, H.B.; Young, M.A. Limited Access to a Dietary Fat Option Affects Ingestive Behavior But Not Body Composition in Male Rats. Physiol. Behav. 1998, 65, 545–553. [Google Scholar] [CrossRef]
- Rodríguez-Arias, M.; Maldonado, C.; Vidal-Infer, A.; Guerri, C.; Aguilar, M.A.; Miñarro, J. Intermittent ethanol exposure increases long-lasting behavioral and neurochemical effects of MDMA in adolescent mice. Psychopharmacology 2011, 218, 429–442. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Rodgers, R.; Cao, B.-J.; Dalvi, A.; Holmes, A. Animal models of anxiety: An ethological perspective. Braz. J. Med. Boil. Res. 1997, 30, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, N.J.; Squire, L.R.; Clark, R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 14515–14520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Prickaerts, J.; Van Staveren, W.; Sik, A.; Ittersum, M.M.-V.; Niewöhner, U.; Van Der Staay, F.; Blokland, A.; De Vente, J. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 2002, 113, 351–361. [Google Scholar] [CrossRef]
- Aguilar, M.; Miñarro, J.; Felipo, V. Chronic Moderate Hyperammonemia Impairs Active and Passive Avoidance Behavior and Conditional Discrimination Learning in Rats. Exp. Neurol. 2000, 161, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, D.; Magriplis, E.; Mitsopoulou, A.; Dimakopoulos, I.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Ntouroupi, T.; Tsaniklidou, S.; et al. Dietary patterns and lifestyle characteristics in adults: Results from the Hellenic National Nutrition and Health Survey (HNNHS). Public Health 2019, 171, 76–88. [Google Scholar] [CrossRef]
- Wojnicki, F.; Johnson, D.; Corwin, R. Access conditions affect binge-type shortening consumption in rats. Physiol. Behav. 2008, 95, 649–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corwin, R.L. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite 2004, 42, 139–142. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Lacerda-Queiroz, N.; Sabino, J.F.; Marques, P.E.; Galvão, I.; Gamba, C.O.; Cassali, G.D.; De Carvalho, L.M.; Silva, D.A.D.S.E.; Versiani, A.; et al. Evaluating the effects of refined carbohydrate and fat diets with acute ethanol consumption using a mouse model of alcoholic liver injury. J. Nutr. Biochem. 2017, 39, 93–100. [Google Scholar] [CrossRef]
- Guzmán-Ruiz, R.; Stucchi, P.; Ramos, M.P.; Sevillano, J.; Somoza, B.; Fernández-Alfonso, M.; Ruiz-Gayo, M. Leptin drives fat distribution during diet-induced obesity in mice. Endocrinol. Nutr. 2012, 59, 354–361. [Google Scholar] [CrossRef]
- Sharma, S.; Fernandes, M.F.; Fulton, S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes. 2013, 37, 1183–1191. [Google Scholar] [CrossRef]
- Leffa, D.D.; Valvassori, S.S.; Varela, R.B.; Lopes-Borges, J.; Daumann, F.; Longaretti, L.M.; Dajori, A.L.F.; Quevedo, J.; Andrade, V.M. Effects of palatable cafeteria diet on cognitive and noncognitive behaviors and brain neurotrophins’ levels in mice. Metab. Brain Dis. 2015, 30, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Takase, K.; Tsuneoka, Y.; Oda, S.; Kuroda, M.; Funato, H. High-fat diet feeding alters olfactory-, social-, and reward-related behaviors of mice independent of obesity. Obesity 2016, 24, 886–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, C.; Spena, G.; Halfon, O.; Boutrel, B. Evidence for a compulsive-like behavior in rats exposed to alternate access to highly preferred palatable food. Addict. Biol. 2014, 19, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, S.; Van Cleef, A.; Davis, J.F. Binge-like intake of HFD attenuates alcohol intake in rats. Physiol. Behav. 2017, 178, 187–195. [Google Scholar] [CrossRef]
- Bake, T.; Murphy, M.; Morgan, D.; Mercer, J. Large, binge-type meals of high fat diet change feeding behaviour and entrain food anticipatory activity in mice. Appetite 2014, 77, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Ganji, A.; Salehi, I.; Nazari, M.; Taheri, M.; Komaki, A. Effects of Hypericum scabrum extract on learning and memory and oxidant/antioxidant status in rats fed a long-term high-fat diet. Metab. Brain Dis. 2017, 32, 1255–1265. [Google Scholar] [CrossRef]
- Shanley, L.J.; Irving, A.J.; Harvey, J. Leptin Enhances NMDA Receptor Function and Modulates Hippocampal Synaptic Plasticity. J. Neurosci. 2001, 21, RC186. [Google Scholar] [CrossRef]
- Naderali, E.K.; Ratcliffe, S.H.; Dale, M.C. Obesity and Alzheimer’s disease: A link between body weight and cognitive function in old age. Am. J. Alzheimer’s Dis. Other Dement. 2009, 24, 445–449. [Google Scholar] [CrossRef]
- Montesinos, J.; Pascual, M.; Pla, A.; Maldonado, C.; Rodriguez-Arias, M.; Miñarro, J.; Guerri, C. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav. Immun. 2015, 45, 233–244. [Google Scholar] [CrossRef]
- Beaudet, G.; Valable, S.; Bourgine, J.; Lelong-Boulouard, V.; Lanfumey, L.; Freret, T.; Paizanis, E. Long-Lasting Effects of Chronic Intermittent Alcohol Exposure in Adolescent Mice on Object Recognition and Hippocampal Neuronal Activity. Alcohol. Clin. Exp. Res. 2016, 40, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Holm-Hansen, S.; Low, J.K.; Zieba, J.; Gjedde, A.; Bergersen, L.H.; Karl, T. Behavioural effects of high fat diet in a mutant mouse model for the schizophrenia risk geneneuregulin 1. Genes Brain Behav. 2016, 15, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cho, H.; Yu, R.T.; Atkins, A.R.; Downes, M.; Evans, R.M. Nuclear receptors rock around the clock. EMBO Rep. 2014, 15, 518–528. [Google Scholar] [CrossRef]
- Roybal, K.; Theobold, D.; Graham, A.; DiNieri, J.A.; Russo, S.J.; Krishnan, V.; Chakravarty, S.; Peevey, J.; Oehrlein, N.; Birnbaum, S.; et al. Mania-like behavior induced by disruption of CLOCK. Proc. Natl. Acad. Sci. USA 2007, 104, 6406–6411. [Google Scholar] [CrossRef]
- McClung, C.A.; Sidiropoulou, K.; Vitaterna, M.; Takahashi, J.S.; White, F.J.; Cooper, D.C.; Nestler, E.J. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc. Natl. Acad. Sci. USA 2005, 102, 9377–9381. [Google Scholar] [CrossRef]
- Stucchi, P.; Gil-Ortega, M.; Merino, B.; Guzmán-Ruiz, R.; Cano, V.; Valladolid-Acebes, I.; Somoza, B.; Le Gonidec, S.; Argente, J.; Valet, P.; et al. Circadian Feeding Drive of Metabolic Activity in Adipose Tissue and not Hyperphagia Triggers Overweight in Mice: Is There a Role of the Pentose-Phosphate Pathway? Endocrinology 2012, 153, 690–699. [Google Scholar] [CrossRef] [Green Version]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Kaneko, K.; Yamada, T.; Tsukita, S.; Takahashi, K.; Ishigaki, Y.; Oka, Y.; Katagiri, H. Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res. 2009, 1263, 58–68. [Google Scholar] [CrossRef]







| Saline | EtOH | |||||
|---|---|---|---|---|---|---|
| Standard Diet | Ad Libitum HFD | Binge HFD | Standard Diet | Ad Libitum HFD | Binge HFD | |
| Liver (g) | 1.83 ± 0.06 | 1.71 ± 0.12 | 2.01 ± 0.08 | 1.75 ± 0.04 | 1.96 ± 0.08 + | 1.79 ± 0.04 |
| Perirrenal WAT (g) | 0.21 ± 0.01 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.23 ± 0.01 | 0.63 ± 0.03 ** | 0.22 ± 0.02 |
| Subcutaneous WAT (g) | 0.28 ± 0.05 | 0.37 ± 0.06 | 0.35 ± 0.04 | 0.28 ± 0.06 | 0.57 ± 0.07 ** | 0.30 ± 0.03 |
| Plasma Glucose (mg/dL) | 247 ± 20 | 193 ± 19 | 240 ± 19 | 213 ± 18 | 220 ± 17 | 227 ± 15 |
| Plasma Insulin (ng/mL) | 0.54 ± 0.21 | 0.29 ± 0.12 | 0.35 ± 0.07 | 0.33 ± 0.07 | 1.17 ± 0.21 *** | 0.26 ± 0.05 |
| Plasma Leptin (ng/mL) | 1.33 ± 0.06 | 2.18 ± 0.48 | 1.69 ± 0.24 | 2.06 ± 0.24 | 3.98 ± 0.67 *** | 1.51 ± 0.23 |
| Saline | EtOH | |||||
|---|---|---|---|---|---|---|
| Standard Diet | Ad Libitum HFD | Binge HFD | Standard Diet | Ad Libitum HFD | Binge HFD | |
| Time in open arms | 58 ± 9 | 78 ± 12 | 122 ± 11 **++ | 76 ± 12 | 95 ± 10 | 117 ± 11 **++ |
| Percentage of time in open arms | 23.9 ± 3.6 | 32.2 ± 4.9 | 47 ± 4 **+ | 33 ± 5 | 36 ± 3 | 45 ± 14 **+ |
| Time in central platform | 52.4 ± 7.2 | 59.1 ± 8.7 | 37 ± 5 | 62 ± 9 | 58 ± 9 | 41 ± 5 |
| Closed Arms | 189.2 ± 11.9 | 162.6 ± 12.9 | 139 ± 11 | 162 ± 15 | 147 ± 15 | 142 ± 8 |
| Entries in open arms | 17.8 ± 1.9 | 22 ± 2.2 | 42 ± 5 **++ | 25 ± 3 | 23 ± 2 | 42 ± 5 **++ |
| Total entries | 50.4 ± 4.1 | 51.5 ± 4.3 | 81 ± 6 **++ | 57 ± 3 | 51 ± 4 | 70 ± 8 **++ |
| Percentage entries in open arms | 35.6 ± 2.9 | 44.5 ± 4.2 | 51 ± 4 | 44 ± 5 | 45 ± 3 | 47 ± 3 |
| Distance traveled | 1688 ± 127 | 1903 ± 187 | 3282 ± 386 ***+++ | 1510 ± 50 | 1653 ± 77 | 2890 ± 445 ***+++ |
| Saline | EtOH | |||||
|---|---|---|---|---|---|---|
| Standard Diet | Ad Libitum HFD | Binge HFD | Standard Diet | Ad Libitum HFD | Binge HFD | |
| DI | 47.8 ± 3.3 | 36.9 ± 9 | 34.1 ± 5.1 | 11.4 ± 6.5 *** | 32.4 ± 7.8 | 33.5 ± 11.2 |
| E1 | 19.8 ± 2.3 | 27.5 ± 2.4 | 16.3 ± 3.3 | 21.5 ± 2.8 | 26.8 ± 2.8 | 16.8 ± 3.2 |
| E2 | 26.1 ± 3.1 | 81.5 ± 5.1 | 14.7 ± 3.3 | 21.6 ± 3.1 | 32.5 ± 5 | 24.3 ± 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Olmo, N.; Blanco-Gandía, M.C.; Mateos-García, A.; Del Rio, D.; Miñarro, J.; Ruiz-Gayo, M.; Rodríguez-Arias, M. Differential Impact of Ad Libitum or Intermittent High-Fat Diets on Bingeing Ethanol-Mediated Behaviors. Nutrients 2019, 11, 2253. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11092253
Del Olmo N, Blanco-Gandía MC, Mateos-García A, Del Rio D, Miñarro J, Ruiz-Gayo M, Rodríguez-Arias M. Differential Impact of Ad Libitum or Intermittent High-Fat Diets on Bingeing Ethanol-Mediated Behaviors. Nutrients. 2019; 11(9):2253. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11092253
Chicago/Turabian StyleDel Olmo, Nuria, M. Carmen Blanco-Gandía, Ana Mateos-García, Danila Del Rio, José Miñarro, Mariano Ruiz-Gayo, and Marta Rodríguez-Arias. 2019. "Differential Impact of Ad Libitum or Intermittent High-Fat Diets on Bingeing Ethanol-Mediated Behaviors" Nutrients 11, no. 9: 2253. https://0-doi-org.brum.beds.ac.uk/10.3390/nu11092253