Herba houttuyniae Extract Benefits Hyperlipidemic Mice via Activation of the AMPK/PGC-1α/Nrf2 Cascade
Abstract
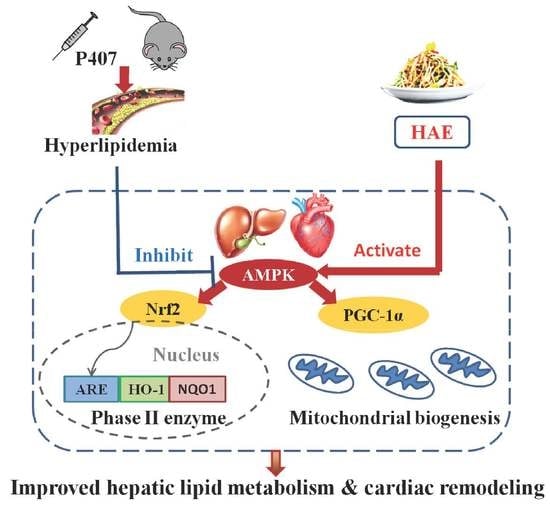
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Treatment
2.3. Biochemical Analysis
2.4. Histological Analysis
2.5. Mitochondrial Complex Activity Analysis
2.6. Western Blot
2.7. Protein Carbonylation Assay
2.8. Real-Time PCR
2.9. Echocardiography
2.10. Statistical Analysis
3. Results
3.1. HAE Improved Hyperlipidemia and Ameliorated Hepatic Lipid Metabolic Disorders
3.2. HAE Improved Cardiac Remodeling
3.3. HAE Activated AMPK in Both the Liver and Heart
3.4. HAE Attenuated Oxidative Stress by Activating the Phase II Enzyme Pathway
3.5. HAE Promoted Mitochondrial Biogenesis and Mitochondrial Complex Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dominiczak, M.H. Hyperlipidaemia and cardiovascular disease--back to basics: Dietary patterns, foods and cardiovascular risk. Curr. Opin. Lipidol. 2011, 22, 509–511. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Klein, B.E.; Myers, C.E.; Howard, K.P.; Klein, R. Serum Lipids and Proliferative Diabetic Retinopathy and Macular Edema in Persons with Long-term Type 1 Diabetes Mellitus: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. JAMA Ophthalmol. 2015, 133, 503–510. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhou, H.; Wu, K.; Lee, S.; Li, R.; Liu, X. PTEN phosphorylation and nuclear export mediate free fatty acid-induced oxidative stress. Antioxid. Redox Signal. 2014, 20, 1382–1395. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Cao, K.; Dong, Z.; Feng, Z.; Liu, J. Combination of beta-glucan and Morus alba L. Leaf Extract Promotes Metabolic Benefits in Mice Fed a High-Fat Diet. Nutrients 2017, 9, 1110. [Google Scholar] [CrossRef] [Green Version]
- Freigang, S.; Ampenberger, F.; Weiss, A.; Kanneganti, T.D.; Iwakura, Y.; Hersberger, M.; Kopf, M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 2013, 14, 1045–1053. [Google Scholar] [CrossRef]
- Liu, C.S.; Kuo, C.L.; Cheng, W.L.; Huang, C.S.; Lee, C.F.; Wei, Y.H. Alteration of the copy number of mitochondrial DNA in leukocytes of patients with hyperlipidemia. Ann. N.Y. Acad. Sci. 2005, 1042, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Hesselink, M.K. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 2004, 53, 1412–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, H.L.; Jung, Y.; Ahn, K.S.; Kwak, H.J.; Um, J.Y. Bitter Orange (Citrus aurantium Linne) Improves Obesity by Regulating Adipogenesis and Thermogenesis through AMPK Activation. Nutrients 2019, 11, 1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.; Kim, Y. Vitamin D Ameliorates Fat Accumulation with AMPK/SIRT1 Activity in C2C12 Skeletal Muscle Cells. Nutrients 2019, 11, 2806. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Sung, M.M.; Zordoky, B.N.; Bujak, A.L.; Lally, J.S.; Fung, D.; Young, M.E.; Horman, S.; Miller, E.J.; Light, P.E.; Kemp, B.E.; et al. AMPK deficiency in cardiac muscle results in dilated cardiomyopathy in the absence of changes in energy metabolism. Cardiovasc. Res. 2015, 107, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Woods, A.; Williams, J.R.; Muckett, P.J.; Mayer, F.V.; Liljevald, M.; Bohlooly, Y.M.; Carling, D. Liver-Specific Activation of AMPK Prevents Steatosis on a High-Fructose Diet. Cell Rep. 2017, 18, 3043–3051. [Google Scholar] [CrossRef] [Green Version]
- Kukidome, D.; Nishikawa, T.; Sonoda, K.; Imoto, K.; Fujisawa, K.; Yano, M.; Motoshima, H.; Taguchi, T.; Matsumura, T.; Araki, E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 2006, 55, 120–127. [Google Scholar] [CrossRef]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef]
- Zimmermann, K.; Baldinger, J.; Mayerhofer, B.; Atanasov, A.G.; Dirsch, V.M.; Heiss, E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis--A role for the unfolded protein response. Free Radic. Biol. Med. 2015, 88, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.; Zheng, A.; Xu, J.; Li, H.; Liu, J.; Peng, Y.; Long, J.; Zou, X.; Li, Y.; Chen, C.; et al. AMPK activation prevents prenatal stress-induced cognitive impairment: Modulation of mitochondrial content and oxidative stress. Free Radic. Biol. Med. 2014, 75, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, J.; Pu, W.; Dong, Z.; Sun, L.; Zang, W.; Gao, F.; Zhang, Y.; Feng, Z.; Liu, J. Punicalagin, an active component in pomegranate, ameliorates cardiac mitochondrial impairment in obese rats via AMPK activation. Sci. Rep. 2015, 5, 14014. [Google Scholar] [CrossRef] [PubMed]
- Greig, F.H.; Ewart, M.A.; McNaughton, E.; Cooney, J.; Spickett, C.M.; Kennedy, S. The hypotensive effect of acute and chronic AMP-activated protein kinase activation in normal and hyperlipidemic mice. Vasc. Pharmacol. 2015, 74, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ma, A.; Zhao, M.; Zhu, H. AMPK activation reduces the number of atheromata macrophages in ApoE deficient mice. Atherosclerosis 2017, 258, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogger, V.C.; Hilmer, S.N.; Sullivan, D.; Muller, M.; Fraser, R.; Le Couteur, D.G. Hyperlipidemia and surfactants: The liver sieve is a link. Atherosclerosis 2006, 189, 273–281. [Google Scholar] [CrossRef]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Wu, H.; Chen, S.; Zhu, Q.; Gao, H.; Yu, X.; Wang, Y.; Su, W.; Yao, X.; et al. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Antivir. Res. 2017, 144, 273–280. [Google Scholar] [CrossRef]
- Hsu, C.C.; Yang, H.T.; Ho, J.J.; Yin, M.C.; Hsu, J.Y. Houttuynia cordata aqueous extract attenuated glycative and oxidative stress in heart and kidney of diabetic mice. Eur. J. Nutr. 2016, 55, 845–854. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yang, J.S.; Chang, W.S.; Tsai, S.C.; Peng, S.F.; Zhou, Y.R. Houttuynia cordata Thunb extract modulates G0/G1 arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J. Biomed. Sci. 2013, 20, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Prasad, S.K.; Krishnamurthy, S.; Hemalatha, S. Antihyperglycemic Activity of Houttuynia cordata Thunb. in Streptozotocin-Induced Diabetic Rats. Adv. Pharmacol. Sci. 2014, 2014, 809438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Yan, C.; Shi, Y.; Cao, K.; Xu, J.; Wang, X.; Chen, C.; Luo, C.; Li, Y.; Gao, J.; et al. Mitochondrial dysfunction in obesity-associated nonalcoholic fatty liver disease: The protective effects of pomegranate with its active component punicalagin. Antioxid. Redox Signal. 2014, 21, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korolenko, T.A.; Tuzikov, F.V.; Johnston, T.P.; Tuzikova, N.A.; Kisarova, Y.A.; Zhanaeva, S.Y.; Alexeenko, T.V.; Zhukova, N.A.; Brak, I.V.; Spiridonov, V.K.; et al. The influence of repeated administration of poloxamer 407 on serum lipoproteins and protease activity in mouse liver and heart. Can. J. Physiol. Pharmacol. 2012, 90, 1456–1468. [Google Scholar] [CrossRef]
- Braun, J.B.S.; Ruchel, J.B.; Adefegha, S.A.; Coelho, A.P.V.; Trelles, K.B.; Signor, C.; Rubin, M.A.; Oliveira, J.S.; Dornelles, G.L.; de Andrade, C.M.; et al. Neuroprotective effects of pretreatment with quercetin as assessed by acetylcholinesterase assay and behavioral testing in poloxamer-407 induced hyperlipidemic rats. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 88, 1054–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Pitzer, A.L.; Chen, Y.; Wang, L.; Li, P.L. Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: Beyond inflammation. Antioxid. Redox Signal. 2015, 22, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.Y.; Shi, T.; Du, G.; Liu, W.; Yin, X.F.; Sun, X.; Pan, Y.; He, Q.Y. iTRAQ-Based Proteomics Revealed the Bactericidal Mechanism of Sodium New Houttuyfonate against Streptococcus pneumoniae. J. Agric. Food Chem. 2016, 64, 6375–6382. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Arch. Pharma. Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef]
- Ma, Q.; Wei, R.; Wang, Z.; Liu, W.; Sang, Z.; Li, Y.; Huang, H. Bioactive alkaloids from the aerial parts of Houttuynia cordata. J. Ethnopharmacol. 2017, 195, 166–172. [Google Scholar] [CrossRef]
- Huh, E.; Kim, H.G.; Park, H.; Kang, M.S.; Lee, B.; Oh, M.S. Houttuynia cordata Improves Cognitive Deficits in Cholinergic Dysfunction Alzheimer’s Disease-Like Models. Biomol. Ther. 2014, 22, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Sun, W.; Wang, X.; Long, J.; Liu, X.; Feng, Z.; Liu, J. Punicalagin attenuates palmitate-induced lipotoxicity in HepG2 cells by activating the Keap1-Nrf2 antioxidant defense system. Mol. Nutr. Food Res. 2016, 60, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Rahman, M.A.; Samad, A. Trace elements in serum from Pakistani patients with acute and chronic ischemic heart disease and hypertension. Clin. Chem. 1984, 30, 644–648. [Google Scholar] [PubMed]
- Ramaiah, S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2007, 45, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Ancellin, N.; Andreelli, F.; Saintillan, Y.; Grondin, P.; Kahn, A.; Thorens, B.; Vaulont, S.; Viollet, B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 2005, 54, 1331–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Xu, C.; Shi, J.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y.; et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut 2018, 67, 2169–2180. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, K.; Lv, W.; Liu, X.; Fan, Y.; Wang, K.; Feng, Z.; Liu, J.; Zang, W.; Xing, L.; Liu, J. Herba houttuyniae Extract Benefits Hyperlipidemic Mice via Activation of the AMPK/PGC-1α/Nrf2 Cascade. Nutrients 2020, 12, 164. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010164
Cao K, Lv W, Liu X, Fan Y, Wang K, Feng Z, Liu J, Zang W, Xing L, Liu J. Herba houttuyniae Extract Benefits Hyperlipidemic Mice via Activation of the AMPK/PGC-1α/Nrf2 Cascade. Nutrients. 2020; 12(1):164. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010164
Chicago/Turabian StyleCao, Ke, Weiqiang Lv, Xuyun Liu, Yingying Fan, Kexin Wang, Zhihui Feng, Jianshu Liu, Weijin Zang, Lianxi Xing, and Jiankang Liu. 2020. "Herba houttuyniae Extract Benefits Hyperlipidemic Mice via Activation of the AMPK/PGC-1α/Nrf2 Cascade" Nutrients 12, no. 1: 164. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12010164