The Impact of Dietary Fucosylated Oligosaccharides and Glycoproteins of Human Milk on Infant Well-Being
Abstract
:1. Introduction
2. Materials and Method
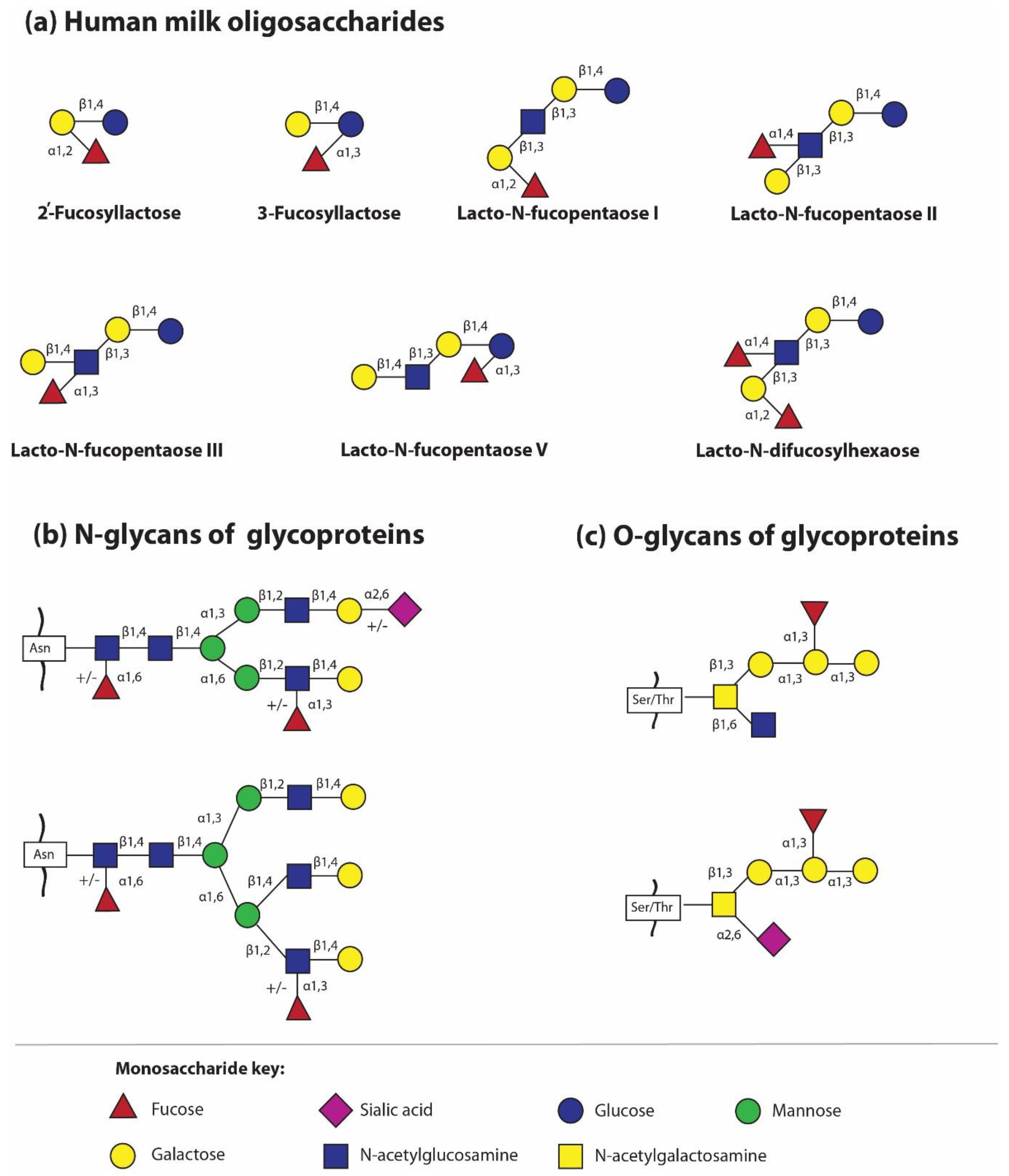
3. Structures and Diversity of HMOs
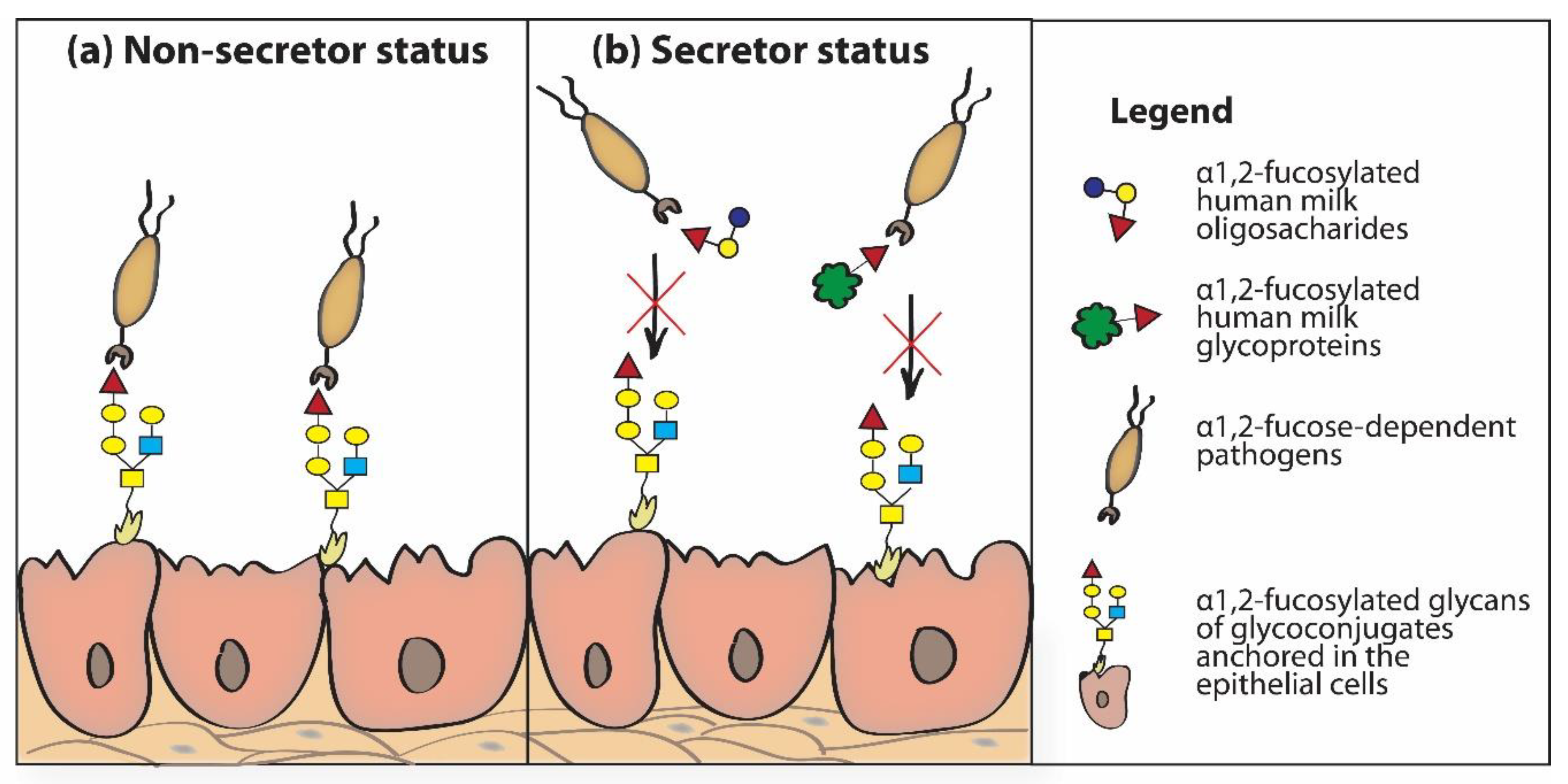
- Secretor (Se+/Le+) – constitutes about 70% of the population,
- Secretor (Se+/Le−) – constitutes about 9% of the population,
- Non-secretor (Se−/Le+) – constitutes about 20% of the population,
- Non-secretor (Se−/Le−) – constitutes about 1% of the population.
4. Fucosylation of Human Milk Glycoproteins
5. Maternal Morbidities and Their Impact on Fucosylation of Dietary HMOs and HMGs
6. Shaping of Infant’s Gut Microbiome by Dietary HMOs
7. Dietary Fucosylated HMOs and HMGs Have Anti-Adhesive Properties
8. Dietary Fucosylated HMOs in Infant’s Blood
9. Dietary Fucosylated HMOs as a Substrate for Synthesis of New Structures
10. Dietary Fucosylated HMOs and HMG Glycans as Immunomodulators
11. Dietary Fucosylated HMOs and Development of Allergy
12. The Impact of Dietary Fucosylated HMOs on Development of Immune Tolerance
13. Dietary Fucosylated HMOs and HMGs in Donor Milk
14. Fucosylated HMOs and Bovine Glycoproteins in Infant Formula
15. Fucosylated HMOs Are Present not only in Human Milk
16. Non-Human Fucosylated Milk Oligosaccharides
17. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2′-FL | 2′-fucosyllactose |
| 3-FL | 3-fucosyllactose |
| AGP | α1- acid glycoprotein |
| DC | dendritic cells |
| DC-SIGN | dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin |
| DFL | difucosyllactose |
| DFpLNnH | difucosyl-para-lacto-N-neohexaose - |
| DSLNT | disialyllacto-N-tetraose |
| FN | fibronectin |
| Fuc | fucose |
| Gal | galactose |
| Glc | glucose |
| GlcNAc | N-acetylglucosamine |
| GOS | galacto-oligosaccharide |
| HMGs | human milk glycoproteins |
| HMOs | human milk oligosaccharides |
| LDFT | lactodifucotetraose |
| LF | lactoferrin |
| LNDFH I | lacto-N-difucohexaose I |
| LNFP I | lacto-N-fucopentaose I |
| LNFP II | lacto-N-fucopentaose II |
| LNFP III | lacto-N-fucopentaose III |
| LNnT | lacto-N-neotetraose |
| LNT | lacto-N-tetraose |
| Neu5Ac | sialic acid |
| S-IgA | secretory immunoglobulin A |
References
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Annales Nestlé 2016, 74, 42–51. [Google Scholar]
- Royle, L.; Roos, A.; Harvey, D.J.; Wormald, M.R.; van Gijlswijk-Janssen, D.; el Redwan, R.M.; Wilson, I.A.; Daha, M.R.; Dwek, R.A.; Rudd, P.M. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 2003, 278, 20140–20153. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgi, G.; Bartke, N.; Wiens, F.; Stahl, B. Functional glycans and glycoconjugates in human milk. Am. J. Clin. Nutr. 2013, 98, 578S–585S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, R.; Cheah, W.Y.; Grinyer, J.; Packer, N. Glycoconjugates in human milk: Protecting infants from disease. Glycobiology 2013, 23, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Bai, Y.; Zhou, J.; Huang, W.; Yan, J.; Tao, J.; Fan, Q.; Liu, Y.; Mei, D.; Yan, Q.; et al. Core fucosylation of maternal milk N-Glycan evokes B cell activation by selectively promoting the I-Fucose metabolism of gut Bifidobacterium spp. and Lactobacillus spp. mBio 2019, 10, e00128-19. [Google Scholar] [CrossRef] [Green Version]
- Craft, K.M.; Townsend, S.D. Mother Knows Best: Deciphering the antibacterial properties of human milk oligosaccharides. Acc. Chem. Res. 2019, 52, 760–768. [Google Scholar] [CrossRef] [Green Version]
- Froehlich, J.W.; Dodds, E.D.; Barboza, M.; McJimpsey, E.L.; Seipert, R.R.; Francis, J.; An, H.J.; Freeman, S.; German, J.B.; Lebrilla, C.B. Glycoprotein expression in human milk during lactation. J. Agric. Food Chem. 2010, 58, 6440–6448. [Google Scholar] [CrossRef] [Green Version]
- Newburg, D.S.; Morelli, K. Human Milk and Infant Intestinal Mucosal Glycans Guide Succession of the Neonatal Intestinal Microbiota. Pediatr. Res. 2015, 77, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, D.L.; Doster, R.S.; Weitkamp, J.-H.; Aronoff, D.M.; Gaddy, J.A.; Townsend, S.D. . Human Milk Oligosaccharides Exhibit Antimicrobial and Antibiofilm Properties Against Group B Streptococcus. ACS Infect. Dis. 2017, 3, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, D.L.; Craft, K.M.; Doster, R.S.; Weitkamp, J.-H.; Aronoff, D.M.; Gaddy, J.A.; Townsend, S.D. Antimicrobial and Antibiofilm Activity of Human Milk Oligosaccharides Against Streptococcus Agalactiae, Staphylococcus Aureus, and Acinetobacter Baumannii. ACS Infect. Dis. 2018, 4, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Townsend, S.D. The Human Milk Glycome as a Defense Against Infectious Diseases: Rationale, Challenges, and Opportunities. ACS Infect. Dis. 2018, 4, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; et al. Human milk oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in infant formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegar, B.; Wibowo, Y.; Basrowi, R.W.; Ranuh, R.G.; Sudarmo, S.M.; Munasir, Z.; Atthiyah, A.F.; Widodo, A.D.; Supriatmo Kadim, M.; Suryawan, A.; et al. The role of two human milk oligosaccharides, 2′-fucosyllactose and lacto-N-neotetraose, in infant nutrition. Pediatr. Gastroenterol. Hepatol Nutr. 2019, 22, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Tonon, M.K.; de Morais, M.B.; Abrão, F.V.; Miranda, A.C.; Morais, T.A.B. Maternal and infant factors associated with human milk oligosaccharides concentrations according to secretor and Lewis phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef] [Green Version]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, J.T.; O’Sullivan, A.; Barile, D.; German, J.B.; Lönnerdal, B.; Slupsky, C.M. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef] [Green Version]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; García-Mantrana, I.; Bertua-Ríos, B.; Martínez-Costa, C.; Borsch, C.; Rudloff, S. Influence of gestational age, secretor, and Lewis blood group status on the oligosaccharide content of human milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Jantscher-Krenn, E. Structure-function relationships of human milk oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; Medina, D.A.; Garrido, D. Human milk oligosaccharides and infant gut bifidobacteria: Molecular strategies for their utilization. Food Microbiol. 2017, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Dallas, D.C.; Martin, W.F.; Strum, J.S.; Zivkovic, A.M.; Smilowitz, J.T.; Underwood, M.A.; Affolter, M.; Lebrilla, C.B.; German, J.B. N-linked glycan profiling of mature human milk by high-performance microfluidic chip liquid chromatography time-of-flight tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 4255–4263. [Google Scholar] [CrossRef] [Green Version]
- Charlwood, J.; Hanrahan, S.; Tyldesley, R.; Langridge, J.; Dwek, M.; Camilleri, P. Use of proteomic methodology for the characterization of human milk fat globular membrane proteins. Anal. Biochem. 2002, 301, 314–324. [Google Scholar] [CrossRef]
- Lis, J.; Orczyk-Pawiłowicz, M.; Kątnik-Prastowska, I. Proteins of human milk involved in immunological processes. Postępy Hig. Med. Dośw. 2013, 67, 529–547. [Google Scholar] [CrossRef]
- Landberg, E.; Huang, Y.; Strömqvist, M.; Mechref, Y.; Hansson, L.; Lundblad, A.; Novotny, M.V.; Påhlsson, P. Changes in glycosylation of human bile-salt-stimulated lipase during lactation. Arch. Biochem. Biophys. 2000, 377, 246–254. [Google Scholar] [CrossRef]
- Orczyk-Pawiłowicz, M.; Hirnle, L.; Berghausen-Mazur, M.; Kątnik-Prastowska, I.M. Lactation stage-related expression of sialylated and fucosylated glycotopes of human milk α-1-acid glycoprotein. Breastfeed. Med. 2014, 9, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. The significance of fucosylated glycoconjugates of human milk in nutrition of newborns and infants. Postępy Hig. Med. Dośw. 2015, 69, 811–829. [Google Scholar] [CrossRef]
- Orczyk-Pawiłowicz, M.; Hirnle, L.; Berghausen-Mazur, M.; Kątnik-Prastowska, I. Terminal glycotope expression on milk fibronectin differs from plasma fibronectin and changes over lactation. Clin. Biochem. 2015, 48, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Autran, C.A.; Schoterman, M.H.; Jantscher-Krenn, E.; Kamerling, J.P.; Bode, L. Sialylated galacto-oligosaccharides and 2′-fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br. J. Nutr. 2016, 116, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M. Sialylated oligosaccharides and glycoconjugates of human milk. The impact on infant and newborn protection, development and well-being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, C.; Kerketta, J.A.; Rao, S.; Patel, S.; Dutt, S.; Arora, K.; Pournami, F.; Bhushan, P. Human milk oligosaccharides: The journey ahead. Int. J. Pediatr. 2019, 2019, 2390240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, S.S. Challenges and pitfalls in human milk oligosaccharide analysis. Nutrients 2019, 11, 2684. [Google Scholar] [CrossRef] [Green Version]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human milk oligosaccharides: Health benefits, potential applications in infant formulas, and pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [Green Version]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Tao, J.; Zhou, J.; Fan, O.; Liu, M.; Hu, Y.; Xu, J.; Zhang, L.; Yuan, J.; Li, W.; et al. Fucosylated Human Milk Oligosaccharides and N-Glycans in the Milk of Chinese Mothers Regulate the Gut Microbiome of Their Breast-Fed Infants During Different Lactation Stages. mSystems 2018, 3, e00206-18. [Google Scholar] [CrossRef] [Green Version]
- Craft, K.M.; Thomas, H.C.; Townsend, S.D. Interrogation of Human Milk Oligosaccharide Fucosylation Patterns for Antimicrobial and Antibiofilm Trends in Group B Streptococcus. ACS Infect. Dis. 2018, 4, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Thomas, H.C.; Townsend, S.D. Sialylated Variants of lacto-N-tetraose Exhibit Antimicrobial Activity Against Group B Streptococcus. Org. Biomol. Chem. 2019, 17, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Day, C.J.; Semchenko, E.A.; Korolik, V. Glycoconjugates play a key role in Campylobacter jejuni infection: Interactions between host and pathogen. Front. Cell Infect. Microbiol. 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Tao, N.; German, J.B.; Grimm, R.; Lebrilla, C.B. Development of an annotated library of neutral human milk oligosaccharides. J. Proteome Res. 2010, 9, 4138–4151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera-Rubio, R.; Kunz, C.; Rudloff, S.; García-Mantrana, I.; Crehuá-Gaudiza, E.; Martínez-Costa, C.; Collado, M.C. Association of maternal secretor status and human milk oligosaccharides with milk microbiota: An observational pilot study. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12, e0171814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leoz, M.L.; Gaerlan, S.C.; Strum, J.S.; Dimapasoc, L.M.; Mirmiran, M.; Tancredi, D.J.; Smilowitz, J.T.; Kalanetra, K.M.; Mills, D.A.; German, J.B.; et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J. Proteome Res. 2012, 11, 4662–4672. [Google Scholar] [CrossRef] [Green Version]
- Austin, S.; De Castro, C.A.; Sprenger, N.; Binia, A.; Affolter, M.; Garcia-Rodenas, C.L.; Beauport, L.; Tolsa, J.F.; Fischer Fumeaux, C.J. Human milk oligosaccharides in the milk of mothers delivering term versus preterm infants. Nutrients 2019, 11, 1282. [Google Scholar] [CrossRef] [Green Version]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; Kimura, K. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br. J. Nutr. 2003, 89, 61–69. [Google Scholar] [CrossRef]
- Newburg, D.S. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J. Anim. Sci. 2009, 87, 26–34. [Google Scholar] [CrossRef]
- Austin, S.; Bénet, T. Quantitative determination of non-lactose milk oligosaccharides. Anal. Chim. Acta 2018, 1010, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Goonatilleke, E.; Huang, J.; Xu, G.; Wu, L.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Human milk proteins and their glycosylation exhibit quantitative dynamic variations during lactation. J. Nutr. 2019, 149, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, J.; Jia, Y.; Yang, Y.; Chen, Q.; Sun, L.; Song, S.; Huang, L.; Wang, Z. Mass spectrometry analysis of changes in human milk n/o-glycopatterns at different lactation stages. J. Agric. Food Chem. 2019, 67, 10702–10712. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Chen, C.; Cline, A.; Newburg, D.S. Human milk oligosaccharides in preterm and term milk. FASEB J. 2016, 1, 673. [Google Scholar]
- Ma, B.; Simala-Grant, J.L.; Taylor, D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology 2006, 16, 158R–184R. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.; Lin, Y.N.; Lin, C.H. Development of fucosyltransferase and fucosidase inhibitors. Chem. Soc. Rev. 2013, 42, 4459–4475. [Google Scholar] [CrossRef]
- Zhu, J.; Dingess, K.A. The functional power of the human milk proteome. Nutrients 2019, 11, 1834. [Google Scholar] [CrossRef] [Green Version]
- Kumazaki, T.; Yoshida, A. Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc. Natl. Acad. Sci. USA 1984, 81, 4193–4197. [Google Scholar] [CrossRef] [Green Version]
- Stahl, B.; Thurl, S.; Henker, J.; Siegel, M.; Finke, B.; Sawatzki, G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv. Exp. Med. Biol. 2001, 501, 299–306. [Google Scholar]
- Totten, S.M.; Zivkovic, A.M.; Wu, S.; Ngyuen, U.; Freeman, S.L.; Ruhaak, L.R.; Darboe, M.K.; German, J.B.; Prentice, A.M.; Lebrilla, C.B. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J. Proteome Res. 2012, 11, 6124–6133. [Google Scholar] [CrossRef]
- Ninonuevo, M.R.; Perkins, P.D.; Francis, J.; Lamotte, L.M.; LoCascio, R.G.; Freeman, S.L.; Mills, D.A.; German, J.B.; Grimm, R.; Lebrilla, C.B. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J. Agric. Food Chem. 2008, 56, 618–626. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Prentice, A.M. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Liu, B.; Newburg, D.S. Human milk glycoproteins protect infants against human pathogens. Breastfeed. Med. 2013, 8, 354–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zhu, S.; Ma, C.; Wang, L.X. Designer α1,6-Fucosidase Mutants Enable Direct Core Fucosylation of Intact N-Glycopeptides and N-Glycoproteins. J. Am. Chem. Soc. 2017, 139, 15074–15087. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Garrido, D.; Mills, D.A.; Barile, D. Hydrolysis of milk gangliosides by infant-gut associated bifidobacteria determined by microfluidic chips and high-resolution mass spectrometry. Electrophoresis 2014, 35, 1742–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, A.D.; Gay, M.C.L.; Trengove, R.D.; Geddes, D.T. Human Milk Lipidomics: Current Techniques and Methodologies. Nutrients 2018, 10, 1169. [Google Scholar] [CrossRef]
- Barboza, M.; Pinzon, J.; Wickramasinghe, S.; Froehlich, J.W.; Moeller, I.; Smilowitz, J.T.; Ruhaak, L.R.; Huang, J.; Lönnerdal, B.; German, J.B.; et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell Proteom. 2012, 11, M111.015248. [Google Scholar] [CrossRef] [Green Version]
- Lis-Kuberka, J.; Orczyk-Pawiłowicz, M.; Królak-Olejnik, B.; Berghausen-Mazur, M.; Barańska, K.; Kątnik-Prastowska, I. Lectin-based analysis of human milk immunoglobulin G fucosylated variants in relation to milk maturation and perinatal risk factors. J. Appl. Biomed. 2018, 16, 232–240. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Kątnik-Prastowska, I.; Berghausen-Mazur, M.; Orczyk-Pawiłowicz, M. Lectin-based analysis of fucosylated glycoproteins of human skim milk during 47 days of lactation. Glycoconj. J. 2015, 32, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Nwosu, C.C.; Aldredge, D.L.; Lee, H.; Lerno, L.A.; Zivkovic, A.M.; German, J.B.; Lebrilla, C.B. Comparison of the human and bovine milk N-glycome via high-performance microfluidic chip liquid chromatography and tandem mass spectrometry. J. Proteome Res. 2012, 11, 2912–2924. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Trivedi, S.S.; Jain, A.; Bhattacharjee, J. Unusual changes in colostrum composition in lactating indian women having medical complications during pregnancy—A pilot study. Indian J. Clin. Biochem. 2002, 17, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.M.D.M.; Cunha, C.C.; Penha-Silva, N.; Abdallah, V.O.S.; Jorge, P.T. Interference of the blood glucose control in the transition between phases I and II of lactogenesis in patients with type 1 diabetes mellitus. Arq. Bras. Endocrinol. Metabol. 2008, 52, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morceli, G.; França, E.L.; Magalhães, V.B.; Damasceno, D.C.; Calderon, I.M.P.; Honorio-França, A.C. Diabetes induced immunological and biochemical changes in human colostrum. Acta Paediatr. 2011, 100, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Bachour, P.; Yafawi, R.; Jaber, F. Effects of smoking, mother’s age, body mass index, and parity number on lipid, protein, and secretory immunoglobulin A concentrations of human milk. Breastfeed. Med. 2012, 7, 179–188. [Google Scholar] [CrossRef]
- Fujimori, M.; França, E.L.; Fiorin, V.; Morais, T.C.; Honorio-França, A.C.; Abreu, L.C. Changes in the biochemical and immunological components of serum and colostrum of overweight and obese mothers. BMC Pregnancy Childbirth 2015, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Massmann, P.F.; França, E.L.; Souza, E.G.; Souza, M.S.; Brune, M.F.S.S.; Honorio-França, A.C. Maternal hypertension induces alterations in immunological factors of colostrum and human milk. Front. Life Sci. 2013, 7, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Amaral, Y.N.D.V.D.; Rocha, D.M.; Silva, L.M.L.D.; Soares, F.V.M.; Moreira, M.E.L. Do maternal morbidities change the nutritional composition of human milk? A systematic review. Cien. Saude Colet. 2019, 24, 2491–2498. [Google Scholar] [CrossRef]
- Peila, C.; Gazzolo, D.; Bertino, E.; Cresi, F.; Coscia, A. Influence of Diabetes during Pregnancy on Human Milk Composition. Nutrients 2020, 12, 185. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, J.T.; Totten, S.M.; Huang, J.; Grapov, D.; Durham, H.A.; Lammi-Keefe, C.J.; Lebrilla, C.; German, J.B. Human milk secretory immunoglobulin a and lactoferrin N-glycans are altered in women with gestational diabetes mellitus. J. Nutr. 2013, 143, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Sjögren, Y.M.; Duchén, K.; Lindh, F.; Björkstén, B.; Sverremark-Ekström, E. Neutral oligosaccharides in colostrum in relation to maternal allergy and allergy development in children up to 18 months of age. Pediatr. Allergy Immunol. 2007, 18, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Salonen, A.; Hickman, B.; Kunz, C.; Sprenger, N.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; de Vos, W.M. Fucosylated oligosaccharides in mother’s milk alleviate the effects of caesarean birth on infant gut microbiota. Sci. Rep. 2018, 8, 13757. [Google Scholar] [CrossRef] [Green Version]
- Fassio, F.; Facioni, M.S.; Guagnini, F. Lactose maldigestion, malabsorption, and intolerance: A comprehensive review with a focus on current management and future perspectives. Nutrients 2018, 10, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human milk oligosaccharides and immune system development. Nutrients 2018, 10, 1038. [Google Scholar] [CrossRef] [Green Version]
- Ashida, H.; Miyake, A.; Kiyohara, M.; Wada, J.; Yoshida, E.; Kumagai, H.; Katayama, T.; Yamamoto, K. Two distinct alpha-L-fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology 2009, 19, 1010–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkhnyatskaya, S.; Ferrari, M.; de Vos, P.; Walvoort, M.T.C. Shaping the infant microbiome with non-digestible carbohydrates. Front. Microbiol. 2019, 10, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, D.; Dallas, D.C.; Mills, D.A. Consumption of human milk glycoconjugates by infant-associated bifidobacteria: Mechanisms and implications. Microbiology 2013, 159, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; Le Parc, A.; Leite Nobrega de Moura Bell, J.M.; Frese, S.A.; Kirmiz, N.; Block, D.E.; Barile, D.; Mills, D.A. Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated Bifidobacteria. Appl. Environ. Microbiol. 2016, 82, 3622–3630. [Google Scholar] [CrossRef] [Green Version]
- Kirmiz, N.; Robinson, R.C.; Shah, I.M.; Barile, D.; Mills, D.A. Milk glycans and their interaction with the infant-gut microbiota. Annu. Rev. Food Sci. Technol. 2018, 9, 429–450. [Google Scholar] [CrossRef]
- Goldman, A.S.; Garza, C.; Schanler, R.J.; Goldblum, R.M. Molecular forms of lactoferrin in stool and urine from infants fed human milk. Pediatr. Res. 1990, 27, 252–255. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.E.; Niñonuevo, M.; Mills, D.A.; Lebrilla, C.B.; German, J.B. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 2007, 51, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, M.; Gotoh, A.; Yoshida, K.; Odamaki, T.; Koguchi, H.; Xiao, J.Z.; Kitaoka, M.; Katayama, T. Varied pathways of infant gut-associated Bifidobacterium to assimilate human milk oligosaccharides: Prevalence of the gene set and its correlation with Bifidobacteria-rich microbiota formation. Nutrients 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sela, D.A.; Li, Y.; Lerno, L.; Wu, S.; Marcobal, A.M.; German, J.B.; Chen, X.; Lebrilla, C.B.; Mills, D.A. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J. Biol. Chem. 2011, 286, 11909–11918. [Google Scholar] [CrossRef] [Green Version]
- Schwab, C.; Gänzle, M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol. Lett. 2011, 315, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Lewis, Z.T.; Totten, S.M.; Smilowitz, J.T.; Popovic, M.; Parker, E.; Lemay, D.G.; Van Tassell, M.L.; Miller, M.J.; Jin, Y.S.; German, J.B.; et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Smith-Brown, P.; Morrison, M.; Krause, L.; Davies, P.S. Mothers secretor status affects development of childrens microbiota composition and function: A pilot study. PLoS ONE 2016, 11, e0161211. [Google Scholar] [CrossRef]
- Solís, G.; de los Reyes-Gavilan, C.G.; Fernández, N.; Margolles, A.; Gueimonde, M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 2010, 16, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatr. 2015, 3, 17. [Google Scholar]
- Van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the gut microbiota by breastfeeding: The gateway to allergy prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Miqdady, M.; Al Mistarihi, J.; Azaz, A.; Rawat, D. Prebiotics in the infant microbiome: The past, present, and future. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.A.E.; O’Neill, I.J.; Kujawska, M.; Gowrinadh Javvadi, S.; Wijeyesekera, A.; Flegg, Z.; Chalklen, L.; Hall, L.J. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 2020, 14, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Taylor, S.L.; Woodman, R.J.; Chen, A.C.; Burr, L.D.; Gordon, D.L.; McGuckin, M.A.; Wesselingh, S.; Rogers, G.B. FUT2 genotype influences lung function, exacerbation frequency and airway microbiota in non-CF bronchiectasis. Thorax 2017, 72, 304–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newburg, D.S.; Grave, G. Recent advances in human milk glycobiology. Pediatr. Res. 2014, 75, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Borewicz, K.; Gu, F.; Saccenti, E.; Arts, I.C.W.; Penders, J.; Thijs, C.; van Leeuwen, S.S.; Lindner, C.; Nauta, A.; van Leusen, E.; et al. Correlating infant faecal microbiota composition and human milk oligosaccharide consumption by microbiota of one-month old breastfed infants. Mol. Nutr. Food Res. 2019, 24, e1801214. [Google Scholar] [CrossRef] [PubMed]
- Cravioto, A.; Tello, A.; Villafán, H.; Ruiz, J.; del Vedovo, S.; Neeser, J.R. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J. Infect. Dis. 1991, 163, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Jennewein, S.; Hüfner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef]
- Coppa, G.V.; Bruni, S.; Zampini, L.; Galeazzi, T.; Facinelli, R.; Capretti, R.; Carlucci, A.; Gabrielli, O. Oligosaccharides of human milk inhibit the adhesion of listeria monocytogenes to Caco 2 cells. Ital. J. Pediatr. 2003, 29, 61–68. [Google Scholar]
- Falk, P.; Roth, K.A.; Borén, T.; Westblom, T.U.; Gordon, J.I.; Normark, S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc. Natl. Acad. Sci. USA 1993, 90, 2035–2039. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, J.; Svennerholm, A.M.; Lindblad, M. Receptor-like glycocompounds in human milk that inhibit classical and El Tor Vibrio cholerae cell adherence (hemagglutination). Infect. Immun. 1983, 39, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Huang, P.; Zhong, W.; Tan, M.; Farkas, T.; Morrow, A.L.; Newburg, D.S.; Ruiz-Palacios, G.M.; Pickering, L.K. Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J. Infect. Dis. 2004, 190, 1850–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Guerrero, M.L.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatr. 2004, 145, 297–303. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Altaye, M.; Chaturvedi, P.; Meinzen-Derr, J.; Guerrero Mde, L.; Morrow, A.L. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2004, 14, 253–263. [Google Scholar] [CrossRef]
- Saeland, E.; de Jong, M.A.; Nabatov, A.A.; Kalay, H.; Geijtenbeek, T.B.; van Kooyk, Y. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol. Immunol. 2009, 46, 2309–2316. [Google Scholar] [CrossRef]
- Taylor, S.L.; McGuckin, M.A.; Wesselingh, S.; Rogers, G.B. Infection’s sweet tooth: How glycans mediate infection and disease susceptibility. Trends Microbiol. 2018, 26, 92–101. [Google Scholar] [CrossRef]
- Pickard, J.M.; Chervonsky, A.V. Intestinal fucose as a mediator of host-microbe symbiosis. J. Immunol. 2015, 194, 5588–5593. [Google Scholar] [CrossRef] [Green Version]
- Duska-McEwen, G.; Senft, A.P.; Ruetschilling, T.L.; Barrett, E.G.; Buck, R.H. Human milk oligosaccharides enhance innate immunity to respiratory syncytial virus and influenza in vitro. Food Nutr. Sci. 2014, 5, 1387–1398. [Google Scholar]
- Laucirica, D.R.; Triantis, V.; Schoemaker, R.; Estes, M.K.; Ramani, S. Milk oligosaccharides inhibit human rotavirus infectivity in MA104 cells. J. Nutr. 2017, 147, 1709–1714. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.Y.; Li, B.; Koike, Y.; Määttänen, P.; Miyake, H.; Cadete, M.; Johnson-Henry, K.C.; Botts, S.R.; Lee, C.; Abrahamsson, T.R.; et al. Human milk oligosaccharides increase mucin expression in experimental necrotizing enterocolitis. Mol. Nutr. Food Res. 2019, 63, e1800658. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Rudloff, S.; Kunz, C.; Strobel, S.; Klein, N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil β2 integrin expression. J. Leukoc. Biol. 2004, 76, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lau, K.; Thon, V.; Autran, C.A.; Jantscher-Krenn, E.; Xue, M.; Li, Y.; Sugiarto, G.; Qu, J.; Mu, S.; et al. Synthetic Disialyl Hexasaccharides Protect Neonatal Rats from Necrotizing Enterocolitis. Angew. Chem. Int. Ed. Engl. 2014, 53, 6687–6691. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides at the interface of maternal-infant health. Breastfeed. Med. 2018, 13, S7–S8. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.L.; Kim, J.H. Human milk and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 34–38. [Google Scholar] [CrossRef]
- Bering, S.B. Human Milk Oligosaccharides to Prevent Gut Dysfunction and Necrotizing Enterocolitis in Preterm Neonates. Nutrients 2018, 10, 1461. [Google Scholar] [CrossRef] [Green Version]
- Gnoth, M.J.; Rudloff, S.; Kunz, C.; Kinne, R.K. Investigations of the in vitro transport of human milk oligosaccharides by a Caco-2 monolayer using a novel high performance liquid chromatography-mass spectrometry technique. J. Biol. Chem. 2001, 276, 34363–34370. [Google Scholar] [CrossRef] [Green Version]
- Goehring, K.C.; Kennedy, A.D.; Prieto, P.A.; Buck, R.H. Direct evidence for the presence of human milk oligosaccharides in the circula-tion of breastfed infants. PLoS ONE 2014, 9, e101692. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Stroble, C.; Underwood, M.A.; Lebrilla, C.B. Detection of milk oligosaccharides in plasma of infants. Anal. Bioanal. Chem. 2014, 406, 5775–5784. [Google Scholar] [CrossRef] [Green Version]
- Triantis, V.; Bode, L.; van Neerven, R.J.J. Immunological effects of human milk oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Delgado-Garcia, J.M.; Buck, R.; Rueda, R.; Martin, M.J. Oral supplementation of 2′-fucosyllactose during lactation improves memory and learning in rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2′-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef] [PubMed]
- Noll, A.J.; Yu, Y.; Lasanajak, Y.; Duska-McEwen, G.; Buck, R.H.; Smith, D.F.; Cummings, R.D. Human DC-SIGN binds specific human milk glycans. Biochem. J. 2016, 473, 1343–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shams-Ud-Doha, K.; Kitova, E.N.; Kitov, P.I.; St-Pierre, Y.; Klassen, J.S. Human milk oligosaccharide specificities of human galectins. Comparison of electrospray ionization mass spectrometry and glycan microarray screening results. Anal. Chem. 2017, 89, 4914–4921. [Google Scholar] [CrossRef]
- Rudloff, S.; Stefan, C.; Pohlentz, G.; Kunz, C. Detection of ligands for selectins in the oligosaccharide fraction of human milk. Eur. J. Nutr. 2002, 41, 85–92. [Google Scholar] [CrossRef]
- Schumacher, G.; Bendas, G.; Stahl, B.; Beermann, C. Human milk oligosaccharides affect P-selectin binding capacities: In vitro investigation. Nutrition 2006, 22, 620–627. [Google Scholar] [CrossRef]
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef]
- Munblit, D.; Peroni, D.; Boix-Amorós, A.; Hsu, P.; Land, B.; Gay, M.; Kolotilina, A.; Skevaki, C.; Boyle, R.; Collado, M.; et al. Human milk and allergic diseases: An unsolved puzzle. Nutrients 2017, 9, 894. [Google Scholar]
- Doherty, A.M.; Lodge, C.J.; Dharmage, S.C.; Dai, X.; Bode, L.; Lowe, A.J. Human milk oligosaccharides and associations with immune-mediated disease and infection in childhood: A systematic review. Front. Pediatr. 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, K.M.; Martin, H.; Oyoshi, M.K. Immunomodulatory effects of breast milk on food allergy. Ann. Allergy Asthma Immunol. 2019, 123, 133–143. [Google Scholar]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seppo, A.E.; Autran, C.A.; Bode, L.; Järvinen, K.M. Human milk oligosaccharides and development of cow’s milk allergy in infants. J. Allergy Clin. Immunol. 2017, 139, 708–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 1767. [Google Scholar] [CrossRef] [PubMed]
- Koning, N.; Kessen, S.F.; Van Der Voorn, J.P.; Appelmelk, B.J.; Jeurink, P.V.; Knippels, L.M.; Garssen, J.; Van Kooyk, Y. Human milk blocks DC-SIGN-pathogen interaction via MUC1. Front. Immunol. 2015, 6, 112. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; van De Worp, W.R.; Stassen, R.; van Maastrigt, C.; Kettelarij, N.; Stahl, B.; Blijenberg, B.; Overbeek, S.A.; Folkerts, G.; Garssen, J.; et al. Human milk oligosaccharides promote immune tolerance via direct interactions with human dendritic cells. Eur. J. Immunol. 2019, 49, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Meier, P.; Patel, A.; Esquerra-Zwiers, A. Donor human milk update: Evidence, mechanisms, and priorities for research and practice. J. Pediatr. 2017, 180, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Hahn, W.H.; Kim, J.; Song, S.; Park, S.; Kang, N.M. The human milk oligosaccharides are not affected by pasteurization and freeze-drying. J. Matern. Fetal Neonatal Med. 2019, 32, 985–991. [Google Scholar] [CrossRef]
- Daniels, B.; Coutsoudis, A.; Autran, C.; Amundson Mansen, K.; Israel-Ballard, K.; Bode, L. The effect of simulated flash heating pasteurisation and Holder pasteurisation on human milk oligosaccharides. Paediatr. Int. Child Health 2017, 37, 204–209. [Google Scholar] [CrossRef]
- Meredith-Dennis, L.; Xu, G.; Goonatilleke, E.; Lebrilla, C.B.; Underwood, M.A.; Smilowitz, J.T. Composition and variation of macronutrients, immune proteins, and human milk oligosaccharides in human milk from nonprofit and commercial milk banks. J. Hum. Lact. 2018, 34, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.; Bridge, R.; Wolf, A.K.; Rich, W.; Kim, J.H.; Bode, L. Human milk oligosaccharide composition differs between donor milk and mother’s own milk in the NICU. J. Hum. Lact. 2014, 30, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Aldredge, D.L.; Geronimo, M.R.; Hua, S.; Nwosu, C.C.; Lebrilla, C.B.; Barile, D. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology 2013, 23, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Townsend, S.D. Synthesis of lacto-N-tetraose. Carbohydr. Res. 2017, 440–441, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yan, X.; Autran, C.A.; Li, Y.; Etzold, S.; Latasiewicz, J.; Robertson, B.M.; Li, J.; Bode, L.; Chen, X. Enzymatic and chemoenzymatic syntheses of disialyl glycans and their necrotizing enterocolitis preventing effects. J. Org.Chem. 2017, 82, 13152–13160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandara, M.D.; Stine, K.J.; Demchenko, A.V. Chemical synthesis of human milk oligosaccharides: Lacto-N-neohexaose (Galβ1 → 4GlcNAcβ1→)2 3,6Galβ1 → 4Glc. Org. Biomol. Chem. 2020, 18, 1747–1753. [Google Scholar] [CrossRef]
- Robinson, R.C. Structures and metabolic properties of bovine milk oligosaccharides and their potential in the development of novel therapeutics. Front. Nutr. 2019, 6, 50. [Google Scholar] [CrossRef]
- Reverri, E.J.; Devitt, A.A.; Kajzer, J.A.; Baggs, G.E.; Borschel, M.W. Review of the clinical experiences of feeding infants formula containing the human milk oligosaccharide 2′-fucosyllactose. Nutrients 2018, 10, 1346. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) Safety of 2-O-fucosyllactose as a novel food ingredient pursuant to regulation (EC) No 258/97. EFSA J. 2015, 13, 4184.
- Van den Abbeele, P.; Duysburgh, C.; Vazquez, E.; Chow, J.; Buck, R.; Marzorati, M. 2′-Fucosyllactose alters the composition and activity of gut microbiota from formula-fed infants receiving complementary feeding in a validated intestinal model. J. Funct. Foods 2019, 61, 103484. [Google Scholar] [CrossRef]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salli, K.; Anglenius, H.; Hirvonen, J.; Hibberd, A.A.; Ahonen, I.; Saarinen, M.T.; Tiihonen, K.; Maukonen, J.; Ouwehand, A.C. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci. Rep. 2019, 9, 13232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parschat, K.; Oehme, A.; Leuschner, J.; Jennewein, S.; Parkot, J. A safety evaluation of mixed human milk oligosaccharides in rats. Food Chem. Toxicol. 2020, 136, 111118. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.S.; Skov, S.H.; Lendal, S.E.; Hornshøj, B.H. Quantifying the human milk oligosaccharides 2′-fucosyllactose and 3-fucosyllactose in different food applications by high-performance liquid chromatography with refractive index detection. J. Food Sci. 2020, 85, 332–339. [Google Scholar] [CrossRef]
- Wang, W.L.; Du, Y.M.; Wang, W.; Conway, L.P.; Cai, Z.P.; Voglmeir, J.; Liu, L. Comparison of the bifidogenic activity of human and bovine milk N-glycome. J. Funct. Foods 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Zhang, L.; van Dijk, A.D.J.; Hettinga, K. An interactomics overview of the human and bovine milk proteome over lactation. Proteome Sci. 2017, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Yang, M.; Yang, N.; Liang, X.; Tao, D.; Liu, B.; Wu, J.; Yue, X. Characterization and comparison of whey N-glycoproteomes from human and bovine colostrum and mature milk. Food Chem. 2019, 276, 266–273. [Google Scholar] [CrossRef]
- Cao, X.; Zheng, Y.; Wu, S.; Yang, N.; Wu, J.; Liu, B.; Ye, W.; Yang, M.; Yue, X. Characterization and comparison of milk fat globule membrane N-glycoproteomes from human and bovine colostrum and mature milk. Food Funct. 2019, 10, 5046–5058. [Google Scholar] [CrossRef]
- Wise, A.; Robertson, B.; Choudhury, B.; Rautava, S.; Isolauri, E.; Salminen, S.; Bode, L. Infants are exposed to human milk oligosaccharides already in utero. Front. Pediatr. 2018, 6, 270. [Google Scholar] [CrossRef] [Green Version]
- Jantscher-Krenn, E.; Aigner, J.; Reiter, B.; Köfeler, H.; Csapo, B.; Desoye, G.; Bode, L.; van Poppel, M.N.M. Evidence of human milk oligosaccharides in maternal circulation already during pregnancy: A pilot study. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E347–E357. [Google Scholar] [CrossRef]
- Hirschmugl, B.; Brandl, W.; Csapo, B.; van Poppel, M.; Köfeler, H.; Desoye, G.; Wadsack, C.; Jantscher-Krenn, E. Evidence of human milk oligosaccharides in cord blood and maternal-to-fetal transport across the placenta. Nutrients 2019, 11, 2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urashima, T.; Taufik, E.; Fukuda, K.; Asakuma, S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci. Biotechnol. Biochem. 2013, 77, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo, J.; Frese, S.A.; Mills, D.A.; Barile, D. Characterization of porcine milk oligosaccharides during early lactation and their relation to the fecal microbiome. J. Dairy Sci. 2016, 99, 7733–7743. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Wang, Z.A.; Wang, B.; Jahan, M.; Wang, Z.; Wynn, P.C.; Du, Y. Characterization of porcine milk oligosaccharides over lactation between primiparous and multiparous female pigs. Sci. Rep. 2018, 8, 4688. [Google Scholar] [CrossRef] [PubMed]
- Leong, A.; Liu, Z.; Almshawit, H.; Zisu, B.; Pillidge, C.; Rochfort, S.; Gill, H. Oligosaccharides in goats’ milk-based infant formula and their prebiotic and anti-infection properties. Br. J. Nutr. 2019, 122, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyrand, M.; Dallas, D.C.; Caillat, H.; Bouvier, F.; Martin, P.; Barile, D. Comparison of milk oligosaccharides between goats with and without the genetic ability to synthesize αs1-casein. Small Rumin. Res. 2013, 113, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Mehra, R.; Barile, D.; Marotta, M.; Lebrilla, C.B.; Chu, C.; German, J.B. Novel high-molecular weight fucosylated milk oligosaccharides identified in dairy streams. PLoS ONE 2014, 9, e96040. [Google Scholar] [CrossRef] [Green Version]
- Mudd, A.T.; Salcedo, J.; Alexander, L.S.; Johnson, S.K.; Getty, C.M.; Chichlowski, M.; Berg, B.M.; Barile, D.; Dilger, R.N. Porcine milk oligosaccharides and sialic acid concentrations vary throughout lactation. Front. Nutr. 2016, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, S.; Lane, J.A.; Mariño, K.; Al Busadah, K.A.; Carrington, S.D.; Hickey, R.M.; Rudd, P.M. A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 2014, 111, 1313–1328. [Google Scholar] [CrossRef]
- Castanys-Muñoz, E.; Martin, M.J.; Prieto, P.A. 2′-fucosyllactose: An abundant, genetically determined soluble glycan present in human milk. Nutr. Rev. 2013, 71, 773–789. [Google Scholar]


| Search Terms Used to Identify Studies on Human Milk | Search Terms Used to Identify Factors Associated with Fucosylated Glycans Content | Search Terms Used to Identify Factors Associated with Infant Well-Being |
|---|---|---|
| human milk AND fucosylation OR fucose AND oligosaccharides OR glycoproteins | secretor status | 2′-fucosyllactose OR 2′-FL |
| human lactation AND fucosylation OR fucose AND oligosaccharides OR glycoproteins | lactation OR milk maturation | microbiota OR microbiome protection OR pathogen adhesion |
| breastfeeding AND fucosylation OR fucose AND oligosaccharides OR glycoproteins | gestational age OR week of delivery | infant formula OR donor milk |
| Human Milk HMOs | Bovine Milk BMOs | Goat Milk GMOs | Porcine Milk PMOs | |
|---|---|---|---|---|
| Concentration | ~20–25 g/L in colostrum and ~5–20 g/L in mature milk [15,16,17] | 1–2 g/L in colostrum and ~0.05–0.1 g/L in mature milk [174,177] | 1.1–1.3 g/L [176] | 23 g/L in colostrum and 5–10 g/L in mature milk [178] |
| Number of identified structures | >200 [5,21,22,23] | over 50 [153] | 29 [176] | 33 [173] |
| Fucosylated structure (% of all structures) | 35–50% [3] | <1% [153] | ok. 20% [176] | 0.89–8.95% [174,178] |
| Concentration of 2′-FL | 2.7 (1.88–4.9) g/L [3] | Absent [179] or present in low amounts [180] | 1.12 mg/L [175] | present [173] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orczyk-Pawiłowicz, M.; Lis-Kuberka, J. The Impact of Dietary Fucosylated Oligosaccharides and Glycoproteins of Human Milk on Infant Well-Being. Nutrients 2020, 12, 1105. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12041105
Orczyk-Pawiłowicz M, Lis-Kuberka J. The Impact of Dietary Fucosylated Oligosaccharides and Glycoproteins of Human Milk on Infant Well-Being. Nutrients. 2020; 12(4):1105. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12041105
Chicago/Turabian StyleOrczyk-Pawiłowicz, Magdalena, and Jolanta Lis-Kuberka. 2020. "The Impact of Dietary Fucosylated Oligosaccharides and Glycoproteins of Human Milk on Infant Well-Being" Nutrients 12, no. 4: 1105. https://0-doi-org.brum.beds.ac.uk/10.3390/nu12041105





