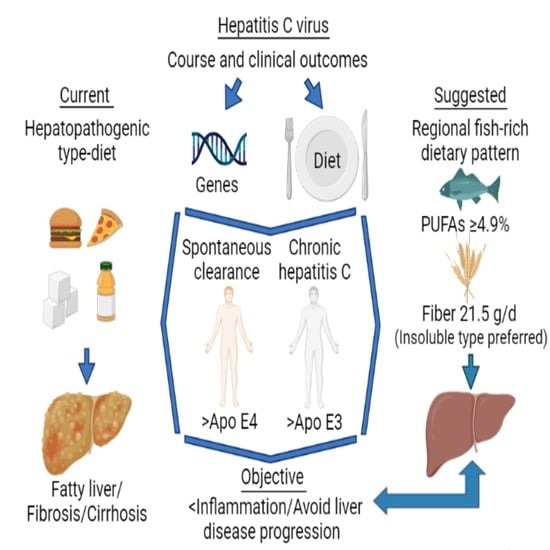
Adherence to a Fish-Rich Dietary Pattern Is Associated with Chronic Hepatitis C Patients Showing Low Viral Load: Implications for Nutritional Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Clinical Data
2.3. Lipid Profile Abnormalities
2.4. APOE Genotyping
2.5. Nutritional Assessment
2.6. Dietary Pattern Analysis
2.7. Statistical Analysis
3. Results
3.1. Demographic, Clinical, and Genetic Characteristics of Patients
3.2. Nutritional Profile of CHC and SC Patients According to APOE Genotype Group
3.3. Identification of Dietary Patterns among CHC and SC Patients
3.4. Association of Nutritional and Biochemical Characteristics with the Clinical Outcome of HCV Infection
3.5. Association of Adherence to Dietary Patterns with HCV-Related Variables
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hepatitis C Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 27 July 2021).
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020, 72, 1605–1616. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Cai, T.; Dufour, J.-F.; Muellhaupt, B.; Gerlach, T.; Heim, M.; Moradpour, D.; Cerny, A.; Malinverni, R.; Kaddai, V.; Bochud, M.; et al. Viral Genotype-Specific Role of PNPLA3, PPARG, MTTP, and IL28B in Hepatitis C Virus-Associated Steatosis. J. Hepatol. 2011, 55, 529–535. [Google Scholar] [CrossRef]
- Grassi, G.; Di Caprio, G.; Fimia, G.M.; Ippolito, G.; Tripodi, M.; Alonzi, T. Hepatitis C Virus Relies on Lipoproteins for Its Life Cycle. World J. Gastroenterol. 2016, 22, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Syed, G.H.; Amako, Y.; Siddiqui, A. Hepatitis C Virus Hijacks Host Lipid Metabolism. Trends Endocrinol. Metab. 2010, 21, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Syed, G.H.; Tang, H.; Khan, M.; Hassanein, T.; Liu, J.; Siddiqui, A. Hepatitis C Virus Stimulates Low-Density Lipoprotein Receptor Expression to Facilitate Viral Propagation. J. Virol. 2014, 88, 2519–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassendine, M.F.; Sheridan, D.A.; Bridge, S.H.; Felmlee, D.J.; Neely, R.D.G. Lipids and HCV. Semin. Immunopathol. 2013, 35, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Yan, P.; Simon, T.G.; Chung, R.T.; Abou-Samra, A.-B.; ERCHIVES Study Team. Changes in Circulating Lipids Level over Time after Acquiring HCV Infection: Results from ERCHIVES. BMC Infect. Dis. 2015, 15, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Fatty Acids, Epigenetic Mechanisms and Chronic Diseases: A Systematic Review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef] [Green Version]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Torres-Castillo, N.; Silva-Gómez, J.A.; Campos-Perez, W.; Barron-Cabrera, E.; Hernandez-Cañaveral, I.; Garcia-Cazarin, M.; Marquez-Sandoval, Y.; Gonzalez-Becerra, K.; Barron-Gallardo, C.; Martinez-Lopez, E. High Dietary ω-6:ω-3 PUFA Ratio Is Positively Associated with Excessive Adiposity and Waist Circumference. Obes. Facts 2018, 11, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.B.; Chisari, F.V. Hepatitis C Virus RNA Replication Is Regulated by Host Geranylgeranylation and Fatty Acids. Proc. Natl. Acad. Sci. USA 2005, 102, 2561–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Saito, H.; Ikeda, M.; Hokari, R.; Kato, N.; Hibi, T.; Miura, S. An Antioxidant Resveratrol Significantly Enhanced Replication of Hepatitis C Virus. World J. Gastroenterol. 2010, 16, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Ikeda, M.; Abe, K.-I.; Dansako, H.; Ohkoshi, S.; Aoyagi, Y.; Kato, N. Comprehensive Analysis of the Effects of Ordinary Nutrients on Hepatitis C Virus RNA Replication in Cell Culture. Antimicrob. Agents Chemother. 2007, 51, 2016–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, J.A.; Jones, K.A.; Flores, R.; Singhania, A.; Woelk, C.H.; Schooley, R.T.; Wyles, D.L. Vitamin D Metabolites Inhibit Hepatitis C Virus and Modulate Cellular Gene Expression. J. Virol. Antivir. Res. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Shibata, C.; Ohno, M.; Otsuka, M.; Kishikawa, T.; Goto, K.; Muroyama, R.; Kato, N.; Yoshikawa, T.; Takata, A.; Koike, K. The Flavonoid Apigenin Inhibits Hepatitis C Virus Replication by Decreasing Mature MicroRNA122 Levels. Virology 2014, 462–463, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Govea-Salas, M.; Rivas-Estilla, A.M.; Rodríguez-Herrera, R.; Lozano-Sepúlveda, S.A.; Aguilar-Gonzalez, C.N.; Zugasti-Cruz, A.; Salas-Villalobos, T.B.; Morlett-Chávez, J.A. Gallic Acid Decreases Hepatitis C Virus Expression through Its Antioxidant Capacity. Exp. Ther. Med. 2016, 11, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Sawada, N.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Shimazu, T.; Yamaji, T.; Takachi, R.; Tanaka, Y.; Mizokami, M.; Tsugane, S.; et al. Consumption of N-3 Fatty Acids and Fish Reduces Risk of Hepatocellular Carcinoma. Gastroenterology 2012, 142, 1468–1475. [Google Scholar] [CrossRef]
- Freire, T.O.; Boulhosa, R.S.S.B.; Oliveira, L.P.M.; de Jesus, R.P.; Cavalcante, L.N.; Lemaire, D.C.; Toralles, M.B.P.; Lyra, L.G.C.; Lyra, A.C. N-3 Polyunsaturated Fatty Acid Supplementation Reduces Insulin Resistance in Hepatitis C Virus Infected Patients: A Randomised Controlled Trial. J. Hum. Nutr. Diet. 2016, 29, 345–353. [Google Scholar] [CrossRef]
- Mueller, T.; Fischer, J.; Gessner, R.; Rosendahl, J.; Böhm, S.; van Bömmel, F. Apolipoprotein E allele frequencies in chronic and self-limited hepatitis C suggest a protective effect of APOE4 in the course of hepatitis C virus infection. Liver Int. 2016, 36, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, H.E.; Mahmoud, M.; Saad, N.E.; Saad-Hussein, A.; Ismail, S.; Thabet, E.H.; Farouk, H.; Kandil, D.; Heiba, A.; Hafez, W. Impact of Apo E gene polymorphism on HCV therapy related outcome in a cohort of HCV Egyptian patients. J. Genet. Eng. Biotechnol. 2018, 16, 47–51. [Google Scholar] [CrossRef]
- Gonzalez-Aldaco, K.; Roman, S.; Torres-Valadez, R.; Ojeda-Granados, C.; Torres-Reyes, L.A.; Panduro, A. Hepatitis C Virus Clearance and Less Liver Damage in Patients with High Cholesterol, Low-Density Lipoprotein Cholesterol and APOE Ε4 Allele. World J. Gastroenterol. 2019, 25, 5826–5837. [Google Scholar] [CrossRef]
- Ronto, R.; Wu, J.H.; Singh, G.M. The Global Nutrition Transition: Trends, Disease Burdens and Policy Interventions. Public Health Nutr. 2018, 21, 2267–2270. [Google Scholar] [CrossRef] [Green Version]
- Betancourt-Nuñez, A.; Márquez-Sandoval, F.; González-Zapata, L.I.; Babio, N.; Vizmanos, B. Unhealthy dietary patterns among healthcare professionals and students in Mexico. BMC Public Health 2018, 18, 1246. [Google Scholar] [CrossRef]
- Cantoral, A.; Contreras-Manzano, A.; Luna-Villa, L.; Batis, C.; Roldán-Valadez, E.A.; Ettinger, A.S.; Mercado, A.; Peterson, K.E.; Téllez-Rojo, M.M.; Rivera, J.A. Dietary Sources of Fructose and Its Association with Fatty Liver in Mexican Young Adults. Nutrients 2019, 11, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, S.; Rivera-Iñiguez, I.; Ojeda-Granados, C.; Sepulveda-Villegas, M.; Panduro, A. Genome-Based Nutrition in Chronic Liver Disease. In Dietary Interventions in Liver Disease—Foods, Nutrients, and Dietary Supplements; Academic Press: Cambridge, MA, USA, 2019; p. 428. [Google Scholar]
- Sepulveda-Villegas, M.; Roman, S.; Rivera-Iñiguez, I.; Ojeda-Granados, C.; Gonzalez-Aldaco, K.; Torres-Reyes, L.A.; Jose-Abrego, A.; Panduro, A. High Prevalence of Nonalcoholic Steatohepatitis and Abnormal Liver Stiffness in a Young and Obese Mexican Population. PLoS ONE 2019, 14, e0208926. [Google Scholar] [CrossRef]
- Bertani, J.P.B.; Alves, B.C.; Azevedo, L.A.; Álvares-da-Silva, M.R.; Dall’Alba, V. Is dietary glycemic load associated with liver fibrosis in hepatitis C? Nutr. Hosp. 2018, 35, 140–147. [Google Scholar] [CrossRef]
- Loguercio, C.; Federico, A.; Masarone, M.; Torella, R.; Blanco del V., C.; Persico, M. The impact of diet on liver fibrosis and on response to interferon therapy in patients with HCV-related chronic hepatitis. Am. J. Gastroenterol. 2008, 103, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Moussa, I.; Day, R.S.; Li, R.; Du, X.L.; Kaseb, A.O.; Jalal, P.K.; Daniel-MacDougall, C.; Hatia, R.I.; Abdelhakeem, A.; Rashid, A.; et al. Dietary Patterns and Hepatocellular Carcinoma Risk among US Adults. Nutrients 2021, 13, 2011. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Torres-Valadez, R.; Roman, S.; Jose-Abrego, A.; Sepulveda-Villegas, M.; Ojeda-Granados, C.; Rivera-Iñiguez, I.; Panduro, A. Early Detection of Liver Damage in Mexican Patients with Chronic Liver Disease. J. Transl. Int. Med. 2017, 5, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-037-SSA2-2012. Para La Prevención, Tratamiento y Control de Las Dislipidemias. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5259329&fecha=13/07/2012 (accessed on 24 May 2021).
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cordero, S.; López-Olmedo, N.; Rodríguez-Ramírez, S.; Barquera-Cervera, S.; Rivera-Dommarco, J.; Popkin, B. Comparing a 7-day diary vs. 24 h-recall for estimating fluid consumption in overweight and obese Mexican women. BMC Public Health 2015, 15, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA. USDA National Nutrient Database for Standard Reference, Release 24; U.S. Department of Agriculture, Agricultural Research Service, USDA Nutrient Data Laboratory: Beltsville, MD, USA, 2011.
- Pérez Lizaur, A.B.; Palacios González, B.; Castro Becerra, A.L.; Flores Galicia, I. Sistema Mexicano de Alimentos Equivalentes, 4th ed.; Cuadernos de Nutrición (Fomento de Nutrición y Salud, A.C.): Mexico City, Mexico, 2011. [Google Scholar]
- Denova-Gutiérrez, E.; Ramírez-Silva, I.; Rodríguez-Ramírez, S.; Jiménez-Aguilar, A.; Shamah-Levy, T.; Rivera-Dommarco, J.A. Validity of a food frequency questionnaire to assess food intake in Mexican adolescent and adult population. Salud Publica México 2016, 58, 617–628. [Google Scholar] [CrossRef]
- Dziuban, C.D.; Shirkey, E.C. When is correlation matrix appropriate for factory analysis? Some decision rules. Psychol. Bull. 1973, 81, 358–361. [Google Scholar] [CrossRef]
- Howard, M.C. Review of Exploratory Factor Analysis Decisions and Overview of Current Practices: What We Are Doing and How Can We Improve? Int. J. Hum.-Comput. Interact. 2016, 32, 51–62. [Google Scholar] [CrossRef]
- O’Connor, B.P. SPSS and SAS Programs for Determining the Number of Components Using Parallel Analysis and Velicer’s MAP Test. Behav. Res. Methods Instrum. Comput. 2000, 32, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Osborne, J.W. Best Practices in Exploratory Factor Analysis, 1st ed.; CreateSpace Independent Publishing: Scotts Valley, CA, USA, 2014; p. 150. [Google Scholar]
- Maasoumy, B.; Vermehren, J. Diagnostics in Hepatitis C: The End of Response-Guided Therapy? J. Hepatol. 2016, 65, S67–S81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Food and Nutrition Board of the Institute of Medicine, The National Academies Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Pawlotsky, J.-M. Hepatitis C Virus Genetic Variability: Pathogenic and Clinical Implications. Clin. Liver Dis. 2003, 7, 45–66. [Google Scholar] [CrossRef]
- Fierro, N.A.; Gonzalez-Aldaco, K.; Torres-Valadez, R.; Martinez-Lopez, E.; Roman, S.; Panduro, A. Immunologic, Metabolic and Genetic Factors in Hepatitis C Virus Infection. World J. Gastroenterol. 2014, 20, 3443–3456. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Beneficial Role of Bioactive Lipids in the Pathobiology, Prevention, and Management of HBV, HCV and Alcoholic Hepatitis, NAFLD, and Liver Cirrhosis: A Review. J. Adv. Res. 2019, 17, 17–29. [Google Scholar] [CrossRef]
- Miyoshi, H.; Moriya, K.; Tsutsumi, T.; Shinzawa, S.; Fujie, H.; Shintani, Y.; Fujinaga, H.; Goto, K.; Todoroki, T.; Suzuki, T.; et al. Pathogenesis of Lipid Metabolism Disorder in Hepatitis C: Polyunsaturated Fatty Acids Counteract Lipid Alterations Induced by the Core Protein. J. Hepatol. 2011, 54, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Granados, C.; Panduro, A.; Rivera-Iñiguez, I.; Sepúlveda-Villegas, M.; Roman, S. A Regionalized Genome-Based Mexican Diet Improves Anthropometric and Metabolic Parameters in Subjects at Risk for Obesity-Related Chronic Diseases. Nutrients 2020, 12, 645. [Google Scholar] [CrossRef] [Green Version]
- Barquera, S.; Rivera, J.A. Obesity in Mexico: Rapid Epidemiological Transition and Food Industry Interference in Health Policies. Lancet Diabetes Endocrinol. 2020, 8, 746–747. [Google Scholar] [CrossRef]
- Ojeda-Granados, C.; Panduro, A.; Gonzalez-Aldaco, K.; Sepulveda-Villegas, M.; Rivera-Iñiguez, I.; Roman, S. Tailoring Nutritional Advice for Mexicans Based on Prevalence Profiles of Diet-Related Adaptive Gene Polymorphisms. J. Pers. Med. 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocke, M.C. Evaluation of methodologies for assessing the overall diet: Dietary quality scores and dietary pattern analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.E.; Bain, V.G.; Ryan, E.A.; Thomson, A.B.R.; Clandinin, M.T. Elevated Lipogenesis and Diminished Cholesterol Synthesis in Patients with Hepatitis C Viral Infection Compared to Healthy Humans. Hepatology 2013, 57, 1697–1704. [Google Scholar] [CrossRef]
- Leu, G.-Z.; Lin, T.-Y.; Hsu, J.T.A. Anti-HCV Activities of Selective Polyunsaturated Fatty Acids. Biochem. Biophys. Res. Commun. 2004, 318, 275–280. [Google Scholar] [CrossRef]
- Hayashi, H.; Tanaka, Y.; Hibino, H.; Umeda, Y.; Kawamitsu, H.; Fujimoto, H.; Amakawa, T. Beneficial Effect of Salmon Roe Phosphatidylcholine in Chronic Liver Disease. Curr. Med. Res. Opin. 1999, 15, 177–184. [Google Scholar] [CrossRef]
- Sheridan, D.A.; Bridge, S.H.; Crossey, M.M.E.; Felmlee, D.J.; Fenwick, F.I.; Thomas, H.C.; Neely, R.D.G.; Taylor-Robinson, S.D.; Bassendine, M.F. Omega-3 Fatty Acids and/or Fluvastatin in Hepatitis C Prior Non-Responders to Combination Antiviral Therapy—A Pilot Randomised Clinical Trial. Liver Int. 2014, 34, 737–747. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Artherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Wolever, T.M.; Rao, A.V.; Hegele, R.A.; Mitchell, S.J.; Ransom, T.P.; Boctor, D.L.; Spadafora, P.J.; Jenkins, A.L.; Mehling, C. Effect on Blood Lipids of Very High Intakes of Fiber in Diets Low in Saturated Fat and Cholesterol. N. Engl. J. Med. 1993, 329, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.Y.; Chen, S.S. Hepatitis C virus and cellular stress response: Implications to molecular pathogenesis of liver diseases. Viruses 2012, 4, 2251–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Sepúlveda, C.A.; Laguna-Meraz, S.; Panduro, A. How far is Mexico from Viral Hepatitis Global Health Sector Strategy 2030 targets. Ann. Hepatol. 2020, 19, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Saldarriaga, O.A.; Dye, B.; Pham, J.; Wanninger, T.G.; Millian, D.; Kueht, M.; Freiberg, B.; Utay, N.; Stevenson, H.L. Comparison of liver biopsies before and after direct-acting antiviral therapy for hepatitis C and correlation with clinical outcome. Sci. Rep. 2021, 11, 14506. [Google Scholar] [CrossRef]
- Rinaldi, L.; Nevola, R.; Franci, G.; Perrella, A.; Corvino, G.; Marrone, A.; Berretta, M.; Morone, M.V.; Galdiero, M.; Giordano, M.; et al. Risk of Hepatocellular Carcinoma after HCV Clearance by Direct-Acting Antivirals Treatment Predictive Factors and Role of Epigenetics. Cancers 2020, 12, 1351. [Google Scholar] [CrossRef]
- Setiono, F.J.; Jock, B.; Trude, A.; Wensel, C.R.; Poirier, L.; Pardilla, M.; Gittelsohn, J. Associations between Food Consumption Patterns and Chronic Diseases and Self-Reported Morbidities in 6 American Indian Communities. Curr. Dev. Nutr. 2019, 3, 69–80. [Google Scholar] [CrossRef]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Base-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Casas, R.; Estruch, R. Dietary Patterns, Foods, Nutrients and Chronic Inflammatory Disorders. Immunome Res. 2016, 12, 122. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, H.; Wang, Z.; Huang, F.; Zhang, X.; Du, W.; Su, C.; Ouyang, Y.; Li, L.; Bai, J.; et al. Trajectories of Dietary Patterns and Their Associations with Overweight/Obesity among Chinese Adults: China Health and Nutrition Survey 1991–2018. Nutrients 2021, 13, 2835. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Granados, C.; Roman, S. Mediterranean Diet or Genome-Based Nutrition Diets in Latin America’s Clinical Practice Guidelines for Managing Chronic Liver Diseases? Ann. Hepatol. 2021, 20, 100291. [Google Scholar] [CrossRef]

| Anti-HCV-Positive Patients | ||||
|---|---|---|---|---|
| Variable | Total Study Group (n = 188) | Chronic Hepatitis C (n = 137) | Spontaneous Clearance (n = 51) | p-Value |
| Demographics/Anthropometrics | ||||
| Age (years) | 49.51 ± 12.0 | 50.9 ± 11.7 | 45.7 ± 12.1 | 0.007 |
| Female/Male n (%) | 106/82 (56.4/43.6) | 78/59 (56.9/43.1) | 28/23 (54.9/45.1) | 0.803 |
| Weight (kg) | 71.4 ± 14.2 | 70.4 ± 14.7 | 74.1 ± 12.5 | 0.120 |
| Height (cm) | 161.5 ± 10.1 | 161.3 ± 10.3 | 162.0 ± 9.6 | 0.689 |
| Body fat mass (kg) | 22.3 ± 9.7 | 21.9 ± 10.2 | 23.2 ± 8.4 | 0.417 |
| Percent body fat (%) | 30.8 ± 9.9 | 12.1 ± 2.7 | 12.7 ± 2.6 | 0.543 |
| BMI (kg/m2) | 27.4 ± 5.2 | 30.5 ± 10.2 | 31.5 ± 9.1 | 0.187 |
| Lipid Profile and Liver Enzymes | ||||
| Total cholesterol (mg/dL) Median (Q1, Q3) | 155.0 ± 49.1 153 (124.0, 183.3) | 142.6 ± 45.1 140.0 (116.0, 166.5) | 191.0 ± 42. 192.0 (157.0, 214.0) | <0.001 a |
| Triglycerides (mg/dL) Median (Q1, Q3) | 139.6 ± 68.5 123.5 (90.0, 181.0) | 128.8 ± 61.1 113.0 (84.0, 166.5) | 171.2 ± 79.1 153.0 (108.0, 210.0) | <0.001 a |
| LDL-c (mg/dL) Median (Q1, Q3) | 93.2 ± 37.8 89.5 (67.0, 111.6) | 84.8 ± 34.7 82.0 (61.5, 100.0) | 118.7 ± 36.1 112.0 (91.0, 136.5) | <0.001 a |
| VLDL-c (mg/dL) Median (Q1, Q3) | 27.7 ± 13.9 24.3 (18.0, 36.0) | 25.9 ± 12.2 22.6 (17.0, 33.8) | 33.0 ± 17.0 29.8 (21.0, 42.0) | 0.002 a |
| HDL-c (mg/dL) | 39.9 ± 15.8 | 38.9 ± 13.4 | 42.7 ± 21.1 | 0.309 |
| Hypercholesterolemia, n (%) | 30 (16.3) | 11 (8.0) | 19 (40.4) | <0.001 |
| Hypertriglyceridemia, n (%) | 68 (37.0) | 44 (32.1) | 24 (51.1) | 0.020 |
| High LDL-c, n (%) | 22 (14.7) | 10 (8.8) | 12 (32.4) | <0.001 |
| Hypoalphalipoproteinemia, n (%) | 108 (71.1) | 81 (71.7) | 27 (69.2) | 0.771 |
| AST (IU/L) Median (Q1,Q3) | 65.7 ± 65.1 49.0 (29.0, 78.0) | 78.0 ± 62.1 61.0 (40.0, 100.0) | 33.7 ± 27.5 27.0 (23.9, 34.8) | <0.001 a |
| ALT (IU/L) Median (Q1,Q3) | 66.4 ± 58.5 45.0 (27.3, 78.0) | 75.7 ± 70.9 57.5 (35.0, 91.8) | 37.5 ± 31.4 27.0 (20.0, 43.5) | <0.001 a |
| GGT (IU/L) Median (Q1,Q3) | 69.9 ± 98.3 39.0 (24.0, 74.0) | 77.5 ± 102.2 50.0 (27.0, 92.0) | 48.7 ± 84.0 24.5 (20.0, 41.0) | <0.001 a |
| APOE Allele Frequency b | ||||
| ε2 | 14 (4.1) | 12 (4.7) | 2 (2.3) | 0.323 |
| ε3 | 297 (86.3) | 224 (87.5) | 73 (83.0) | 0.284 |
| ε4 | 33 (9.6) | 20 (7.8) | 13 (14.7) | 0.056 |
| Variables | Chronic Hepatitis C | Spontaneous Clearance | RDA Values | ||||
|---|---|---|---|---|---|---|---|
| E2 (n = 10) | E3 (n = 100) | E4 (n = 18) | E2 (n = 2) | E3 (n = 30) | E4 (n = 12) | ||
| Anthropometrics | |||||||
| Weight (kg) | 69.1 ± 5.5 | 70.5 ± 14.9 | 72.7 ± 18.3 | 75.9 ± 26.7 | 70.5 ± 9.6 | 80.0 ± 16.9 | — |
| Body fat (%) | 26.6 ± 10.9 | 31.4 ± 10.1 | 29.9 ± 10.6 | 30.1 ± 0.8 | 30.2 ± 9.0 | 31.8 ± 6.8 | — |
| BMI (kg/m2) | 26.1 ± 3.9 | 27.3 ± 5.2 | 27.8 ± 5.5 | 28.0 ± 4.6 | 27.0 ± 3.7 | 29.4 ± 4.9 | — |
| Macronutrients | |||||||
| Total energy (kcal) | 2061 ± 446 | 2082 ± 696 | 1900 ± 510 | 2903 ± 4 | 2070 ± 470 | 2135 ± 414 | — |
| Proteins (%) | 15.7 ± 5.9 | 17.1 ± 4.0 | 16.1 ± 4.2 | 14.0 ± 1.4 | 16.0 ± 4.6 | 19.0 ± 4.9 | 15–20 |
| Total fat (%) | 24.5 ± 10.0 | 29.1 ± 8.2 | 26.3 ± 7.9 | 24.0 ± 7.1 | 29.2 ± 9.3 | 29.9 ± 3.9 | 25–30 |
| SFA (%) | 5.9 ± 4.4 | 7.8 ± 3.3 | 6.8 ± 2.7 | 6.5 ± 3.5 | 8.2 ± 3.6 | 7.1 ± 3.2 | <7 |
| MUFA (%) | 8.1 ± 5.1 | 9.9 ± 4.0 | 8.9 ± 4.0 | 8.0 ± 1.4 | 9.6 ± 4.6 | 11.2 ± 6.9 | 10–15 |
| PUFA (%) | 3.8 ± 1.5 | 4.8 ± 2.2 | 4.5 ± 1.7 | 4.0 ± 0.0 | 4.6 ± 2.1 | 5.6 ± 3.2 | 7–10 |
| Cholesterol (mg/dL) | 217.8 ± 166.3 | 311.4 ± 251.6 | 239.5 ± 155.0 | 125.0 ± 0.0 | 300.1 ± 248.9 | 334.5 ± 207.3 | <200 |
| Carbohydrates (%) | 61.4 ± 12.9 | 55.9 ± 10.0 | 59.8 ± 9.9 | 64.0 ± 7.1 | 57.1 ± 12.4 | 52.6 ± 3.8 | 50–55 |
| Fiber (g/day) | 18.9 ± 8.3 | 25.3 ± 12.7 | 19.3 ± 8.3 | 28.3 ± 0.0 | 24.2 ± 12.7 | 25.2 ± 1.5 | 25–38 |
| Sugar (g/day) | 29.0 ± 20.3 | 36.2 ± 26.4 | 48.8 ± 39.0 | 40.1 ± 0.0 | 48.6 ± 48.6 | 32.8 ± 24.9 | <30 |
| Micronutrients a | |||||||
| Vitamin A (μg/day) | 1407.9 ± 2288.7 | 1045.1 ± 1138.9 | 1493.6 ± 1689.7 | 831 ± 0.0 | 950.1 ± 777.8 | 1184.1 ± 535.2 | 900 |
| Vitamin E (mg/day) | 1.7 ± 1.0 | 2.6 ± 2.0 | 2.0 ± 1.6 | 3.8 ± 0.0 | 2.4 ± 2.2 | 2.7 ± 1.9 | 15 |
| Folates (μg/day of DFE) | 98.3 ± 101.4 | 189.2 ± 148.1 | 157.5 ± 80.2 | 90.0 ± 0.0 | 173.6 ± 147.9 | 366.1 ± 360.6 | 300–600 |
| Thiamin (mg/day) | 1.2 ± 0.5 | 1.3 ± 0.7 | 1.2 ± 0.6 | 2.0 ± 0.0 | 1.3 ± 0.6 | 1.7 ± 1.3 | 1.1–1.2 |
| Pyridoxine (mg/day) | 1.0 ± 0.3 | 1.3 ± 0.8 | 1.5 ± 0.9 | 0.6 ± 0.0 | 1.2 ± 0.9 | 1.8 ± 1.4 | 1.7 |
| Cobalamin (μg/day) | 3.1 ± 3.3 | 3.5 ± 2.5 | 2.7 ± 2.1 | 4.4 ± 0.0 | 2.5 ± 1.9 | 4.8 ± 3.3 | 2.4 |
| Iron (mg/day) | 14.3 ± 6.3 | 16.2 ± 7.2 | 13.4 ± 4.4 | 27.3 ± 0.0 | 14.2 ± 5.6 | 20.5 ± 12.6 | 8–18 |
| Selenium (μg/day) | 30.4 ± 27.5 | 42.6 ± 37.8 | 26.6 ± 19.7 | 47.0 ± 0.0 | 42.7 ± 36.4 | 59.1 ± 73.5 | 55 |
| Chronic Hepatitis C Patients | ||||
|---|---|---|---|---|
| Variables | Reference Values | Low Viral Load (n = 36) | High Viral Load (n = 101) | p-Value |
| Anthropometrics | ||||
| Body fat (%) | — | 30.7 ± 10.3 | 30.4 ± 10.3 | 0.882 |
| BMI (kg/m2) | — | 27.7 ± 5.3 | 26.9 ± 5.5 | 0.458 |
| Macro- and Micronutrients | Ref [46] | |||
| Total energy (kcal) Median (Q1, Q3) | — | 1926 ± 483 1852 (1562, 2322) | 2126 ± 713 1970 (1685, 2555) | 0.233 a |
| Proteins (%) | 15–20 | 17.7 ± 4.5 | 16.5 ± 4.0 | 0.138 |
| Total fat (%) | 25–30 | 30.9 ± 8.2 | 27.5 ± 8.0 | 0.030 |
| SFA (%) | <7 | 8.0 ± 3.3 | 7.5 ± 3.3 | 0.449 |
| MUFA (%) | 10–15 | 10.4 ± 4.1 | 9.4 ± 4.0 | 0.216 |
| PUFA (%) | 7–10 | 5.4 ± 2.7 | 4.4 ± 1.9 | 0.030 |
| Cholesterol (mg/dL) Median (Q1, Q3) | <200 | 316.0 ± 231.8 229.5 (147.5, 431.0) | 278.5 ± 236.5 199 (107.0, 415.8) | 0.239 a |
| Carbohydrates (%) | 50–55 | 54.1 ± 9.6 | 57.9 ± 10.2 | 0.052 |
| Fiber (g/day) | 25–38 | 19.8 ± 9.5 | 26.3 ± 13.8 | 0.016 |
| Sugar (g/day) | <30 | 33.3 ± 28.9 | 39.0 ± 26.6 | 0.314 |
| Vitamin A (μg/day) | 900 | 1050.0 ± 1268.1 | 1099.4 ± 1314.0 | 0.844 |
| Vitamin E (mg/day) | 15 | 2.3 ± 1.9 | 2.4 ± 1.9 | 0.742 |
| Laboratory Data | Ref [33] | |||
| Glucose (mg/dL) Median (Q1, Q3) | ≤100 | 103.5 ± 34.8 96.5 (84.3, 109.8) | 114.4 ± 58.7 97.0 (87.0, 116.5) | 0.334 a |
| ALT (IU/L) Median (Q1, Q3) | ≤42 | 53.3 ± 34.7 42.5 (29.5, 70.3) | 83.8 ± 78.6 61.5 (37.0, 103.8) | 0.011 a |
| AST (IU/L) Median (Q1, Q3) | ≤54 | 62.7 ± 40.1 52.5 (35.5, 77.5) | 83.6 ± 67.7 65.0 (41.0, 107.0) | 0.083 a |
| GGT (IU/L) Median (Q1, Q3) | ≤30 | 44.0 ± 27.4 33.5 (26.3, 64.5) | 88.8 ± 115.1 54.0 (28.0, 110.0) | 0.013 a |
| Platelets (×103/μL) | 150–450 | 161.7 ± 116.4 | 150.0 ± 71.0 | 0.590 |
| Albumin (g/dL) | 3.4–5.4 | 3.4 ± 0.6 | 3.6 ± 0.6 | 0.047 |
| T. bilirubin (mg/dL) Median (Q1, Q3) | 0.1–1.2 | 1.2 ± 0.9 1.0 (0.6, 1.5) | 1.3 ± 1.6 0.9 (0.6, 1.4) | 0.935 a |
| Dietary Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p-Value | OR | 95 % CI | p-Value | |
| Total fat (%) | 0.949 | 0.904–0.996 | 0.032 | |||
| PUFA (%) | 0.805 | 0.668–0.969 | 0.022 | 0.804 | 0.656–0.986 | 0.036 |
| Carbohydrates (%) | 1.040 | 0.999–1.082 | 0.055 | |||
| Fiber (g/day) | 1.050 | 1.008–1.093 | 0.019 | 1.049 | 1.007–1.092 | 0.021 |
| HCV Viral Load | Variable | Cut-Off | AUC | p-Value | Sensitivity, % | Specificity, % |
|---|---|---|---|---|---|---|
| Low | PUFA (%) | ≥4.9 | 0.624 | 0.032 | 61.8 | 56.6 |
| High | Fiber (g/day) | ≥21.5 | 0.633 | 0.026 | 61.4 | 69.7 |
| Dietary Patterns | HCV-Related Variables | ||||
|---|---|---|---|---|---|
| Adherence Tertile | PUFA ≥4.9% | Fiber ≥21.5 g/day | Low Viral Load | High Viral Load | |
| Meat and soft drinks, DP1 | T1 | 19 (54.4) | 20 (57.1) | 13 (36.1) | 23 (63.9) |
| T2 | 15 (42.9) | 12 (46.7) | 5 (13.9) | 31 (86.1) | |
| T3 | 14 (40.0) | 16 (50.0) | 10 (27.8) | 26 (72.2) | |
| Processed animal and fried foods, DP2 | T1 | 16 (38.1) | 19 (45.2) | 8 (19.0) | 34 (81.0) |
| T2 | 15 (45.5) | 16 (57.1) | 11 (32.4) | 23 (67.6) | |
| T3 | 17 (56.7) | 15 (55.6) | 9 (28.1) | 23 (71.9) | |
| Mexican-healthy DP3 | T1 | 17 (50.0) | 20 (62.5) | 10 (28.6) | 25 (71.4) |
| T2 | 15 (41.7) | 14 (42.4) | 11 (29.7) | 26 (70.3) | |
| T3 | 16 (45.7) | 16 (50.0) | 7 (19.4) | 29 (80.6) | |
| Fish-rich DP4 | T1 | 11 (29.7) | 24 (68.6) | 6 (16.2) | 31 (83.8) |
| T2 | 16 (42.1) | 13 (39.4) | 10 (25.0) | 30 (75.0) | |
| T3 | 21 (70.0) a | 13 (44.8) b | 12 (38.7) c | 19 (61.3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda-Granados, C.; Panduro, A.; Gonzalez-Aldaco, K.; Rivera-Iñiguez, I.; Campos-Medina, L.; Roman, S. Adherence to a Fish-Rich Dietary Pattern Is Associated with Chronic Hepatitis C Patients Showing Low Viral Load: Implications for Nutritional Management. Nutrients 2021, 13, 3337. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103337
Ojeda-Granados C, Panduro A, Gonzalez-Aldaco K, Rivera-Iñiguez I, Campos-Medina L, Roman S. Adherence to a Fish-Rich Dietary Pattern Is Associated with Chronic Hepatitis C Patients Showing Low Viral Load: Implications for Nutritional Management. Nutrients. 2021; 13(10):3337. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103337
Chicago/Turabian StyleOjeda-Granados, Claudia, Arturo Panduro, Karina Gonzalez-Aldaco, Ingrid Rivera-Iñiguez, Liliana Campos-Medina, and Sonia Roman. 2021. "Adherence to a Fish-Rich Dietary Pattern Is Associated with Chronic Hepatitis C Patients Showing Low Viral Load: Implications for Nutritional Management" Nutrients 13, no. 10: 3337. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103337