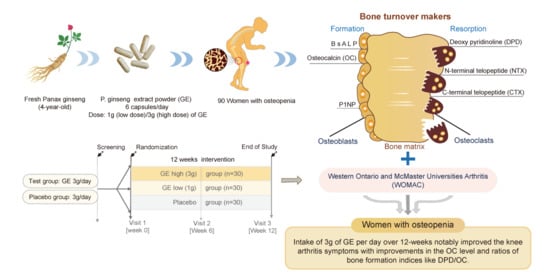
Effect of Ginseng Extracts on the Improvement of Osteopathic and Arthritis Symptoms in Women with Osteopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Supplements
2.2. Participants
2.3. Study Design
2.4. Outcome Measurements
2.4.1. Primary Outcomes
2.4.2. Secondary Outcomes
2.5. Safety Measurements
2.6. Evaluation of Diet and Physical Activity
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Diet Intake and Physical Activity
3.3. Efficacy Evaluation
3.3.1. Primary Outcome
3.3.2. Secondary Outcome
3.4. Safety and Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.-C.; Shin, D.-H.; Lee, S.-Y.; Im, J.-A.; Lee, D.-C. Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med. J. 2010, 51, 857–863. [Google Scholar] [CrossRef] [Green Version]
- Parfitt, A.; Pødenphant, J.; Villanueva, A.; Frame, B. Metabolic bone disease with and without osteomalacia after intestinal bypass surgery: A bone histomorphometric study. Bone 1985, 6, 211–220. [Google Scholar] [CrossRef]
- Lee, H.-S.; Baek, I.-G.; Hong, E.-S. Effects of nutrients intakes on development of osteoporosis in Korean postmenopausal women. J. Korean Diet. Assoc. 1996, 2, 38–48. [Google Scholar]
- Choi, H. Development and conservation of the female skeleton. J. Korean Soc. Osteoporos. 2008, 6, 1–7. [Google Scholar]
- Oh, Y.; Kim, H.; Ock, M.; Cha, H. Effects of menopausal symptoms, ysterectomy and bilateral salpingoophorectomy on bone mineral contents using dual energy X-ray absorptiometry. J. Korean Soc. Osteoporos. 2009, 7, 103–110. [Google Scholar]
- Bergström, I.; Parini, P.; Gustafsson, S.A.; Andersson, G.; Brinck, J. Physical training increases osteoprotegerin in postmenopausal women. J. Bone Miner. Metab. 2012, 30, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Kohrt, W.M. Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: A meta-analysis of randomized controlled trials. BMC Musculoskelet. Disord. 2012, 13, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-Z.; Shin, A.; Lee, J.; Myung, S.-K.; Kim, J. The beneficial effect of leisure-time physical activity on bone mineral density in pre-and postmenopausal women. Calcif. Tissue Int. 2012, 91, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kling, J.M.; Clarke, B.L.; Sandhu, N.P. Osteoporosis prevention, screening, and treatment: A review. J. Women’s Health 2014, 23, 563–572. [Google Scholar] [CrossRef]
- Rizzoli, R.; Bonjour, J. Determinants of peak bone mass and mechanisms of bone loss. Osteoporos. Int. 1999, 9, S17. [Google Scholar] [CrossRef]
- Xu, J.; Lombardi, G.; Jiao, W.; Banfi, G. Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: An overview of systematic reviews and meta-analyses. Sports Med. 2016, 46, 1165–1182. [Google Scholar] [CrossRef]
- Cornall, L.M.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell. Physiol. Biochem. 2011, 28, 949–958. [Google Scholar] [CrossRef]
- Kim, M.-G.; Ha, K.-C.; Back, H.-I.; Kim, S.-Y.; Kim, J.-W.; Kim, K.-Y.; Cho, H.-R.; Chae, H.-J.; Chae, S.-W.; Kim, D.-S. Effects of Polycan on bone metabolism in healthy perimenopausal women: A 12-week randomized, double-blind, placebo-controlled study. Korean J. Clin. Pharm. 2011, 21, 297–304. [Google Scholar]
- Park, S.Y.; Suh, Y.S.; Yoon, S.J.; Park, H.M.; Hur, M. Relationship between initial biochemical bone markers and change of bone mineral density of postmenopausal women with short-term hormone replacement therapy. Korean J. Obstet. Gynecol. 1999, 42, 1972–1979. [Google Scholar]
- Majidi, Z.; Ansari, M.; Maghbooli, Z.; Ghasemi, A.; Ebrahimi, S.S.S.; Hossein-Nezhad, A.; Emamgholipour, S. Oligopin® Supplementation Mitigates Oxidative Stress in Postmenopausal Women with Osteopenia: A Randomized, Double-blind, Placebo-Controlled Trial. Phytomedicine 2021, 81, 153417. [Google Scholar] [CrossRef]
- Na, H.-B.; Kim, H.-J.; Park, J. Effects of calcium supplementation and exercise on bone mineral density in middle-aged women. J. Nutr. Health 2002, 35, 962–969. [Google Scholar]
- Jeong, Y.; Kim, J.; Seo, J.; Kim, G. Effects of liquefied calcium supplement on bone mineral density in middle-aged women. J. Korean Soc. Food Sci. Nutr. 2004, 33, 995–999. [Google Scholar]
- Ahn, E.-K.; Kim, K.-S.; Park, S.-M.; Choi, J.-Y. Effect of DM-BSF on Bone Mineral Density in Postmenopausal Women: Randomized, Double-Blind, Placebo-Controlled, Parallel Trial. Korean J. Fam. Pr. 2015, 5, 421–429. [Google Scholar]
- CHOI, K.t. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.-Y. Korean Ginseng: Composition, Processing, and Health Benefits. In Korean Functional Foods; CRC Press: Boca Raton, FL, USA, 2018; pp. 233–256. [Google Scholar]
- Cui, Y.; Shu, X.-O.; Gao, Y.-T.; Cai, H.; Tao, M.-H.; Zheng, W. Association of ginseng use with survival and quality of life among breast cancer patients. Am. J. Epidemiol. 2006, 163, 645–653. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Park, S.-H.; Choi, E.-K.; Jung, S.-J.; Pyo, M.K.; Chae, S.-W. A randomized, double-blind, placebo-controlled pilot study to assess the effects of protopanaxadiol saponin–enriched ginseng extract and pectinase-processed ginseng extract on the prevention of acute respiratory illness in healthy people. J. Ginseng Res. 2020, 44, 697–703. [Google Scholar] [CrossRef]
- Park, E.-K.; Shin, Y.-W.; Lee, H.-U.; Kim, S.-S.; Lee, Y.-C.; Lee, B.-Y.; Kim, D.-H. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264. 7 cells induced by lipopolysaccharide. Biol. Pharm. Bull. 2005, 28, 652–656. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Mir, I.; Rubio, E.; Morales-Olivas, F.J.; Palop-Larrea, V. Transient ischemic attack secondary to hypertensive crisis related to Panax ginseng. Ann. Pharmacother. 2004, 38, 1970. [Google Scholar] [CrossRef]
- Huong, N.T.T.; Murakami, Y.; Tohda, M.; Watanabe, H.; Matsumoto, K. Social isolation stress-induced oxidative damage in mouse brain and its modulation by majonoside-R2, a Vietnamese ginseng saponin. Biol. Pharm. Bull. 2005, 28, 1389–1393. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-G.; Cho, J.-H.; Yoo, S.-R.; Lee, J.-S.; Han, J.-M.; Lee, N.-H.; Ahn, Y.-C.; Son, C.-G. Antifatigue effects of Panax ginseng CA Meyer: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e61271. [Google Scholar]
- Schliebs, R.; Liebmann, A.; Bhattacharya, S.K.; Kumar, A.; Ghosal, S.; Bigl, V. Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem. Int. 1997, 30, 181–190. [Google Scholar] [CrossRef]
- Ji, Y.C.; Kim, Y.B.; Park, S.W.; Hwang, S.N.; Min, B.K.; Hong, H.J.; Kwon, J.T.; Suk, J.S. Neuroprotective effect of ginseng total saponins in experimental traumatic brain injury. J. Korean Med. Sci. 2005, 20, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-H.; Oh, M.-R.; Choi, E.-K.; Kim, M.-G.; Ha, K.-C.; Lee, S.-K.; Kim, Y.-G.; Park, B.-H.; Kim, D.-S.; Chae, S.-W. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J. Ginseng Res. 2014, 38, 239–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pipat, C.; Kanyarat, R. Effects of standardized ginseng extract and exercise training on aerobic and anaerobic exercise capacities in humans. J. Ginseng Res. 1995, 19, 93–100. [Google Scholar]
- Lee, S.-T.; Chu, K.; Sim, J.-Y.; Heo, J.-H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef]
- Jung, S.-J.; Hwang, J.-H.; Park, S.-H.; Choi, E.-K.; Ha, K.-C.; Baek, H.-I.; Shin, D.-G.; Seo, J.-H.; Chae, S.-W. A 12-week, randomized, double-blind study to evaluate the efficacy and safety of liver function after using fermented ginseng powder (GBCK25). Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Li, Y.-Q.; Li, S.-P.; Ma, L.; Ding, L.-J.; Ji, H. Alleviation of ovariectomy-induced osteoporosis in rats by Panax notoginseng saponins. J. Nat. Med. 2010, 64, 336–345. [Google Scholar] [CrossRef]
- Avsar, U.; Karakus, E.; Halici, Z.; Bayir, Y.; Bilen, H.; Aydin, A.; Avsar, U.; Ayan, A.; Aydin, S.; Karadeniz, A. Prevention of bone loss by Panax ginseng in a rat model of inflammation-induced bone loss. Cell. Mol. Biol. 2013, 59, 1835–1841. [Google Scholar]
- Park, C.-M.; Kim, H.-M.; Kim, D.H.; Han, H.-J.; Noh, H.; Jang, J.-H.; Park, S.-H.; Chae, H.-J.; Chae, S.-W.; Ryu, E.K. Ginsenoside re inhibits osteoclast differentiation in mouse bone marrow-derived macrophages and zebrafish scale model. Mol. Cells 2016, 39, 855. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-M.; Kim, D.H.; Han, H.-J.; Park, C.-M.; Ganipisetti, S.R.; Valan Arasu, M.; Kim, Y.O.; Park, C.G.; Kim, B.-Y.; Soung, N.-K. Ginsenoside Re promotes osteoblast differentiation in mouse osteoblast precursor MC3T3-E1 cells and a Zebrafish model. Molecules 2017, 22, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-J.; Park, K.-H.; Kim, D.-H.; Chae, H.-J.; Sung, G.-H.; Kim, Y.-O. In vitro assessments of bone microcomputed tomography in an aged male rat model supplemented with Panax ginseng. Saudi J. Biol. Sci. 2018, 25, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, D.Y.; Lee, S.E.; Nam, K.Y.; Hwang, G.B.; Kim, H.D.; Lee, J.W.; Choi, J.H.; Ahn, Y.S.; Kim, S.Y. Evaluation on extraction conditions and HPLC analysis method for ginsenosides in Panax ginseng. Korean J. Med. Crop. Sci. 2016, 24, 47–54. [Google Scholar] [CrossRef]
- Song, Y.J.; Paik, H.Y. Effect of dietary, biochemical and other factors on bone mineral density change for 2 years in Korean college women. Korean J. Nutr. 2003, 36, 175–182. [Google Scholar]
- Bellamy, N. Pain assessment in osteoarthritis: Experience with the WOMAC osteoarthritis index. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 1989; pp. 14–17. [Google Scholar]
- Armstrong, T.; Bull, F. Development of the world health organization global physical activity questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Kim, M.R.; Kwon, D.J.; Chung, G.W.; Kim, J.H.; Lim, Y.T.; Kim, E.J. The correlation of combined biochemical markers of bone turnover for bone mineral density in postmenopausal women. Korean J. Obstet. Gynecol. 2001, 43, 1066–1070. [Google Scholar]
- Åkesson, K.; Vergnaud, P.; Delmas, P.; Obrant, K. Serum osteocalcin increases during fracture healing in elderly women with hip fracture. Bone 1995, 16, 427–430. [Google Scholar]
- Choi, S.H.; Lee, S.Y.; Kim, Y.J. The pattern of urinary deoxypyridinoline and serum osteocalcin across menopausal transition in women. J. Korean Acad. Fam. Med. 2000, 21, 1552–1559. [Google Scholar]
- McLaren, A.; Hordon, L.; Bird, H.; Robins, S. Urinary excretion of pyridinium crosslinks of collagen in patients with osteoporosis and the effects of bone fracture. Ann. Rheum. Dis. 1992, 51, 648–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, K.; Larsson, A.; Simonsson, B.; Turesson, I.; Westin, J.; Ljunghall, S. Evaluation of bone disease in multiple myeloma: A comparison between the resorption markers urinary deoxypyridinoline/creatinine (DPD) and serum ICTP, and an evaluation of the DPD/osteocalcin and ICTP/osteocalcin ratios. Eur. J. Haematol. 1999, 62, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.A.; Anderson, J.; Gallagher, P.N., Jr. Intakes of calcium, phosphorus, and protein, and physical-activity level are related to radial bone mass in young adult women. Am. J. Clin. Nutr. 1993, 58, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-S.; Jin, M.H.; Koh, H.-M. Association between Serum Osteocalcin Levels and Metabolic Syndrome according to the Menopausal Status of Korean Women. J. Korean Med. Sci. 2021, 36, e56. [Google Scholar] [CrossRef]
- Garnero, P.; Piperno, M.; Gineyts, E.; Christgau, S.; Delmas, P.; Vignon, E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: Relations with disease activity and joint damage. Ann. Rheum. Dis. 2001, 60, 619–626. [Google Scholar] [CrossRef] [Green Version]



| Ingredients | Contents (%) | ||
|---|---|---|---|
| Ginseng Extract Powder 1 g (Low Dose) | Ginseng Extract Powder 3 g (High Dose) | Placebo | |
| Ginseng powder | 33.3 | >99.0 | |
| Silicon dioxide | 0.50 | 0.5 | |
| Magnesium stearate | 0.24 | 0.5 | 0.37 |
| Microcrystalline cellulose | 65.53 | 98.98 | |
| Ginseng flavor | 0.397 | 0.60 | |
| Caramel coloring | 0.026 | 0.04 | |
| Gardenia yellow coloring | 0.007 | 0.01 | |
| Total | 100 | 100 | 100 |
| Variables | GE Low (1 g) Group (n = 30) | GE High (3 g) Group (n = 30) | Placebo Group (n = 30) | Total (n = 90) | p-Value 1 |
|---|---|---|---|---|---|
| Age, years | 55.40 ± 3.17 | 53.93 ± 2.82 | 54.63 ± 3.02 | 54.66 ± 3.03 | 0.174 |
| Menopause age (years) | 50.27 ± 3.94 | 48.57 ± 4.04 | 49.43 ± 4.01 | 49.42 ± 4.01 | 0.263 |
| Menopause duration (months) | 61.87 ± 40.05 | 64.00 ± 55.78 | 62.40 ± 50.76 | 62.76 ± 48.75 | 0.985 |
| Number of pregnancies | 2.07 ± 0.58 | 2.20 ± 0.81 | 2.23 ± 0.86 | 2.17 ± 0.75 | 0.668 |
| Height (cm) | 156.57 ± 4.25 | 155.20 ± 6.22 | 155.37 ± 5.50 | 155.71 ± 5.36 | 0.565 |
| Weight (kg) | 58.45 ± 7.80 | 59.52 ± 9.31 | 57.21 ± 7.97 | 58.39 ± 8.35 | 0.569 |
| Body mass index (kg/m2) | 23.85 ± 3.01 | 24.65 ± 3.03 | 23.66 ± 2.69 | 24.05 ± 2.91 | 0.379 |
| Drinking (yes/no) | 10/20 | 9/21 | 12/18 | 31/59 | 0.709 2 |
| Alcohol consumption (units/week) | 1.14 ± 1.18 | 1.10 ± 1.79 | 2.31 ± 2.68 | 1.58 ± 2.06 | 0.305 |
| Current, smoker (yes/no) | 1.14 ± 1.18 | 1.10 ± 1.79 | 2.31 ± 2.68 | 1.58 ± 2.06 | 0.305 |
| VBD(T-score) | −2.25 ± 0.75 | −1.99 ± 0.64 | −1.96 ± 0.71 | −2.07 ± 0.71 | 0.207 |
| FBD(T-score) | −1.40 ± 0.88 | −1.15 ± 0.67 | −1.22 ± 0.77 | −1.26 ± 0.78 | 0.433 |
| OC (ng/mL) | 21.3 ± 5.72 | 18.93 ± 3.72 | 21.11 ± 6.34 | 20.4 ± 5.38 | 0.173 |
| DPD (nM DPD/mM crea) | 7.43 ± 1.33 | 7.33 ± 1.52 | 8.29 ± 4.74 | 7.67 ± 2.91 | 0.410 |
| DPD/OC ratio | 0.37 ± 0.1 | 0.40 ± 0.09 | 0.42 ± 0.26 | 0.39 ± 0.17 | 0.538 |
| CTX (ng/mL) | 0.48 ± 0.11 | 0.49 ± 0.16 | 0.45 ± 0.16 | 0.48 ± 0.15 | 0.534 |
| OC/CTX ratio | 44.48 ± 11.39 | 41.10 ± 12.16 | 49.92 ± 15.51 | 45.16 ± 13.02 | 0.078 |
| NTX (ng/mL) | 15.58 ± 2.88 | 15.95 ± 4.05 | 15.93 ± 4.03 | 15.82 ± 3.66 | 0.914 |
| Ca (mg/dL) | 9.45 ± 0.36 | 9.35 ± 0.27 | 9.33 ± 0.25 | 9.38 ± 0.3 | 0.260 |
| P (mg/dL) | 3.67 ± 0.35 | 3.81 ± 0.33 | 3.72 ± 0.49 | 3.74 ± 0.39 | 0.421 |
| BsALP (μg/L) | 16.59 ± 5.06 | 15.91 ± 3.56 | 17.23 ± 5.93 | 16.55 ± 4.88 | 0.601 |
| P1 NP (pg/mL) | 61.23 ± 13.69 | 59.10 ± 14.97 | 58.18 ± 15.98 | 59.51 ± 14.78 | 0.737 |
| WOMAC index | 10.54 ± 12.75 | 14.7 ± 12.63 | 11.2 ± 11.87 | 8 ± 9.12 | 0.096 |
| GE Low (1 g) Group (n = 28) | GE High (3 g) Group (n = 30) | Placebo Group (n = 27) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Week | 12 Week | Diff | p-Value 1 | 0 Week | 12 Week | Diff | p-Value 1 | 0 Week | 12 Week | Diff | p-Value 1 | p-Value 2 | |
| Calorie (kcal) | 1681.30 ± 434.63 | 1609.45 ± 464.11 | −71.85 ± 508.34 | 0.461 | 1522.94 ± 495.53 | 1557.56 ± 354.22 | 34.62 ± 502.06 | 0.708 | 1584.74 ± 426.59 | 1478.53 ± 409.78 | −106.21 ± 416.01 | 0.196 | 0.510 |
| Carbohydrate (g) | 267.60 ± 78.58 | 267.70 ± 78.98 | 0.10 ± 90.58 | 0.995 | 244.00 ± 73.47 | 250.91 ± 62.14 | 6.90 ± 80.63 | 0.643 | 257.09 ± 64.63 | 246.56 ± 57.16 | −10.54 ± 66.31 | 0.417 | 0.712 |
| Lipid (g) | 40.34 ± 20.35 | 34.78 ± 16.60 | −5.56 ± 18.90 | 0.131 | 36.26 ± 20.88 | 38.64 ± 20.24 | 2.38 ± 28.35 | 0.649 | 39.90 ± 23.55 | 33.58 ± 18.65 | −6.31 ± 25.34 | 0.207 | 0.333 |
| Protein (g) | 67.21 ± 21.18 | 63.08 ± 23.21 | −4.13 ± 26.26 | 0.412 | 61.62 ± 27.87 | 60.28 ± 21.67 | −1.34 ± 25.26 | 0.774 | 54.70 ± 20.10 | 55.33 ± 20.06 | 0.63 ± 22.62 | 0.886 | 0.774 |
| Fiber (g) | 23.13 ± 7.98 | 24.94 ± 8.54 | 1.81 ± 11.71 | 0.421 | 22.26 ± 11.93 | 23.14 ± 9.14 | 0.89 ± 10.08 | 0.634 | 21.89 ± 7.00 | 21.17 ± 5.86 | −0.72 ± 7.40 | 0.616 | 0.635 |
| Vitamin D (μg) | 5.39 ± 8.21 | 4.90 ± 6.41 | −0.49 ± 9.41 | 0.779 | 5.84 ± 6.82 | 4.61 ± 5.15 | −1.23 ± 8.24 | 0.419 | 3.82 ± 6.84 | 3.02 ± 4.80 | −0.80 ± 8.57 | 0.630 | 0.948 |
| Vitamin C (mg) | 115.22 ± 65.40 | 135.33 ± 68.58 | 20.11 ± 89.52 | 0.236 | 109.74 ± 87.04 | 102.07 ± 68.86 | −6.68 ± 72.24 | 0.930 | 91.67 ± 51.49 | 118.41 ± 51.16 | 26.73 ± 58.88 | 0.026 | 0.635 |
| Calcium (mg) | 512.22 ± 147.42 | 499.36 ± 221.46 | −12.85 ± 237.3 | 0.773 | 587.51 ± 346.72 | 522.00 ± 250.61 | −65.51 ± 318.93 | 0.270 | 490.13 ± 247.10 | 547.06 ± 267.21 | 56.94 ± 316.30 | 0.358 | 0.635 |
| Phosphorus (mg) | 1072.28 ± 236.41 | 1006.02 ± 341.98 | −66.26 ± 389.43 | 0.367 | 1009.47 ± 463.45 | 971.41 ± 336.96 | −38.05 ± 378.95 | 0.587 | 869.79 ± 321.65 | 876.61. ± 320.15 | 6.86 ± 332.16- | 0.915 | 0.635 |
| MET (min/week) | 2490.00 ± 2122.15 | 3002.14 ± 3423.55 | 512.14 ± 3697.90 | 0.470 | 3002.67 ± 3203.45 | 4350.00 ± 4649.10 | 1347.33 ± 4114.29 | 0.083 | 1822.67 ± 1418.57 | 4309.63 ± 5266.72 | 2486.96 ± 5241.23 | 0.021 * | 0.251 |
| GE Low (1 g) Group (n = 28) | GE High (3 g) Group (n = 30) | Placebo Group (n = 27) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Week | 12 Week | Diff | p-Value 1 | 0 Week | 12 Week | Diff | p-Value 1 | 0 Week | 12 Week | Diff | p-Value 1 | p-Value 2 | p-Value 3 | |
| Osteocalcin (ng/mL) | 21.30 ± 5.72 | 22.05 ± 5.77 | 0.74 ± 4.97 a | 0.436 | 18.93 ± 3.72 | 23.45 ± 5.81 | 4.52 ± 4.42 b | <0.0001 *** | 21.11 ± 6.34 | 21.50 ± 8.16 | 0.39 ± 4.31 a | 0.643 | 0.001 ** | <0.001 *** |
| DPD (nM DPD/mM crea) | 7.43 ± 1.33 | 6.86 ± 1.42 | −0.58 ± 1.57 | 0.061 | 7.33 ± 1.52 | 7.23 ± 2.04 | −0.11 ± 1.61 | 0.722 | 8.29 ± 4.74 | 7.85 ± 5.53 | −0.44 ± 1.59 | 0.166 | 0.510 | 0.564 |
| DPD/OC ratio | 0.37 ± 0.10 | 0.33 ± 0.12 | −0.04 ± 0.10 ab | 0.057 | 0.40 ± 0.09 | 0.32 ± 0.08 | −0.08 ± 0.05 a | <0.0001 *** | 0.42 ± 0.26 | 0.41 ± 0.37 | −0.01 ± 0.15 b | 0.772 | 0.046 * | 0.027 * |
| CTX (ng/mL) | 0.48 ± 0.11 | 0.48 ± 0.12 | 0.00 ± 0.08 | 0.802 | 0.49 ± 0.16 | 0.49 ± 0.14 | 0.00 ± 0.10 | 0.961 | 0.45 ± 0.16 | 0.47 ± 0.17 | 0.02 ± 0.11 | 0.411 | 0.683 | 0.918 |
| OC/CTX ratio | 44.48 ± 11.39 | 46.83 ± 11.08 | 2.32 ± 10.82 a | 0.268 | 41.10 ± 12.16 | 48.72 ± 11.51 | 7.49 ± 12.89 b | 0.004 ** | 49.92 ± 15.51 | 47.42 ± 11.78 | −2.37 ± 12.54 a | 0.329 | 0.013 * | 0.021 * |
| NTX (ng/mL) | 15.58 ± 2.88 | 15.40 ± 2.58 | −0.17 ± 1.63 | 0.580 | 15.95 ± 4.05 | 15.39 ± 3.74 | −0.56 ± 2.17 | 0.170 | 15.93 ± 4.03 | 15.83 ± 4.42 | −0.09 ± 2.37 | 0.837 | 0.664 | 0.757 |
| Ca (mg/dL) | 9.45 ± 0.36 | 9.42 ± 0.28 | −0.03 ± 0.36 a | 0.679 | 9.35 ± 0.27 | 9.53 ± 0.32 | 0.18 ± 0.24 b | <0.001 *** | 9.33 ± 0.25 | 9.38 ± 0.25 | 0.06 ± 0.29 ab | 0.335 | 0.034 * | 0.017 * |
| p (mg/dL) | 3.67 ± 0.35 | 3.68 ± 0.37 | 0.00 ± 0.41 | 0.964 | 3.81 ± 0.33 | 3.72 ± 0.39 | −0.08 ± 0.36 | 0.221 | 3.72 ± 0.49 | 3.81 ± 0.51 | 0.09 ± 0.43 | 0.274 | 0.263 | 0.173 |
| BsALP(μg/L) | 16.59 ± 5.06 | 16.31 ± 3.67 | −0.28 ± 4.05 | 0.719 | 15.91 ± 3.56 | 15.07 ± 3.63 | −0.84 ± 2.46 | 0.071 | 17.23 ± 5.93 | 17.07 ± 5.84 | −0.16 ± 2.87 | 0.771 | 0.689 | 0.720 |
| P1NP (pg/mL) | 61.23 ± 13.69 | 58.46 ± 12.98 | −2.77 ± 6.63 | 0.036* | 59.10 ± 14.97 | 59.01 ± 16.15 | −0.09 ± 7.31 | 0.947 | 58.18 ± 15.98 | 61.29 ± 21.89 | 3.11 ± 13.91 | 0.256 | 0.087 | 0.170 |
| GE Low (1 g) Group (n = 28) | GE High (3 g) Group (n = 30) | Placebo Group (n = 27) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Week | 12 Week | Diff | p-Value 1 | 0 Week | 12 Week | Diff | p-Value 1 | 0 Week | 12 Week | Diff | p-Value 1 | p-Value 2 | p-Value 3 | |
| WOMAC index | ||||||||||||||
| Pain | ||||||||||||||
| Q1 | 0.36 ± 0.68 | 0.57 ± 0.96 | 0.21 ± 0.74 | 0.136 | 0.50 ± 0.68 | 0.40 ± 0.56 | −0.10 ± 0.71 | 0.448 | 0.22 ± 0.42 | 0.26 ± 0.53 | 0.04 ± 0.52 | 0.713 | 0.205 | 0.184 |
| Q2 | 0.50 ± 0.69 | 0.68 ± 0.77 | 0.18 ± 0.67 | 0.170 | 0.87 ± 1.01 | 0.77 ± 0.73 | −0.10 ± 0.92 | 0.558 | 0.48 ± 0.51 | 0.63 ± 0.63 | 0.15 ± 0.66 | 0.256 | 0.319 | 0.248 |
| Q3 | 0.18 ± 0.39 | 0.21 ± 0.57 | 0.04 ± 0.58 ab | 0.746 | 0.57 ± 0.97 | 0.30 ± 0.65 | −0.27 ± 0.98 a | 0.147 | 0.11 ± 0.42 | 0.33 ± 0.62 | 0.22 ± 0.42 b | 0.011 * | 0.037 * | 0.122 |
| Q4 | 0.18 ± 0.39 | 0.36 ± 0.62 | 0.18 ± 0.55 | 0.096 | 0.33 ± 0.76 | 0.30 ± 0.47 | −0.03 ± 0.85 | 0.832 | 0.30 ± 0.61 | 0.37 ± 0.69 | 0.07 ± 0.62 | 0.537 | 0.507 | 0.459 |
| Q5 | 0.18 ± 0.39 | 0.29 ± 0.60 | 0.11 ± 0.50 | 0.264 | 0.33 ± 0.66 | 0.40 ± 0.62 | 0.07 ± 0.74 | 0.625 | 0.19 ± 0.48 | 0.37 ± 0.69 | 0.19 ± 0.74 | 0.202 | 0.796 | 0.740 |
| Stiffness | ||||||||||||||
| Q1 | 0.46 ± 0.74 | 0.64 ± 0.78 | 0.18 ± 0.61 | 0.134 | 0.73 ± 0.78 | 0.60 ± 0.81 | −0.13 ± 1.01 | 0.475 | 0.33 ± 0.55 | 0.44 ± 0.75 | 0.11 ± 0.64 | 0.376 | 0.283 | 0.249 |
| Q2 | 0.46 ± 0.69 | 0.64 ± 0.91 | 0.18 ± 0.82a | 0.259 | 1.03 ± 0.81 | 0.63 ± 0.72 | −0.40 ± 0.77 b | 0.008 ** | 0.48 ± 0.70 | 0.52 ± 0.70 | 0.04 ± 0.76 a | 0.802 | 0.016 * | 0.011* |
| Physical function | ||||||||||||||
| Q1 | 0.64 ± 0.91 | 0.75 ± 0.93 | 0.11 ± 0.79 | 0.477 | 0.67 ± 0.88 | 0.73 ± 0.87 | 0.07 ± 0.91 | 0.690 | 0.59 ± 0.64 | 0.52 ± 0.58 | −0.07 ± 0.62 | 0.537 | 0.669 | 0.892 |
| Q2 | 0.68 ± 0.82 | 0.68 ± 0.82 | 0.00 ± 0.67 | >0.999 | 0.77 ± 0.86 | 0.63 ± 0.76 | −0.13 ± 0.68 | 0.293 | 0.52 ± 0.58 | 0.59 ± 0.64 | 0.07 ± 0.62 | 0.537 | 0.482 | 0.296 |
| Q3 | 0.39 ± 0.79 | 0.61 ± 0.83 | 0.21 ± 0.79 | 0.161 | 0.77 ± 0.86 | 0.67 ± 0.80 | −0.10 ± 1.06 | 0.610 | 0.3 ± 0.55 | 0.63 ± 0.79 | 0.30 ± 0.78 | 0.058 | 0.209 | 0.320 |
| Q4 | 0.46 ± 0.74 | 0.50 ± 0.69 | 0.04 ± 0.58 | 0.746 | 0.57 ± 0.73 | 0.53 ± 0.86 | −0.03 ± 1.07 | 0.865 | 0.37 ± 0.56 | 0.41 ± 0.75 | 0.04 ± 0.76 | 0.802 | 0.934 | 0.801 |
| Q5 | 0.54 ± 0.74 | 0.46 ± 0.58 | −0.07 ± 0.81 | 0.646 | 0.63 ± 0.85 | 0.53 ± 0.68 | −0.10 ± 0.99 | 0.586 | 0.30 ± 0.67 | 0.37 ± 0.79 | 0.07 ± 0.92 | 0.678 | 0.749 | 0.687 |
| Q6 | 0.43 ± 0.74 | 0.50 ± 0.75 | 0.07 ± 0.72 | 0.602 | 0.67 ± 0.88 | 0.47 ± 0.73 | −0.20 ± 1.06 | 0.312 | 0.30 ± 0.47 | 0.44 ± 0.70 | 0.15 ± 0.66 | 0.256 | 0.261 | 0.127 |
| Q7 | 0.50 ± 0.75 | 0.50 ± 0.75 | 0.00 ± 0.47 | >0.999 | 0.53 ± 0.73 | 0.60 ± 0.77 | 0.07 ± 0.87 | 0.677 | 0.33 ± 0.55 | 0.52 ± 0.75 | 0.19 ± 0.62 | 0.134 | 0.596 | 0.715 |
| Q8 | 0.32 ± 0.72 | 0.54 ± 0.84 | 0.21 ± 0.63 | 0.083 | 0.50 ± 0.82 | 0.6 ± 0.81 | 0.10 ± 0.84 | 0.522 | 0.26 ± 0.45 | 0.26 ± 0.53 | 0.00 ± 0.55 | >0.999 | 0.521 | 0.602 |
| Q9 | 0.32 ± 0.61 | 0.39 ± 0.79 | 0.07 ± 0.54 | 0.490 | 0.33 ± 0.55 | 0.33 ± 0.55 | 0.00 ± 0.69 | >0.999 | 0.19 ± 0.40 | 0.37 ± 0.69 | 0.19 ± 0.56 | 0.096 | 0.512 | 0.437 |
| Q10 | 0.61± 0.79 | 0.46 ± 0.74 | −0.14 ± 0.65 | 0.255 | 0.63 ± 0.76 | 0.53 ± 0.57 | −0.10 ± 0.80 | 0.501 | 0.22 ± 0.51 | 0.48 ± 0.85 | 0.26 ± 0.66 | 0.050 | 0.076 | 0.106 |
| Q11 | 0.25 ± 0.59 | 0.43 ± 0.79 | 0.18 ± 0.55 | 0.096 | 0.33 ± 0.55 | 0.37 ± 0.61 | 0.03 ± 0.72 | 0.801 | 0.19 ± 0.40 | 0.41 ± 0.8 | 0.22 ± 0.64 | 0.083 | 0.506 | 0.541 |
| Q12 | 0.29 ± 0.71 | 0.39 ± 0.74 | 0.11 ± 0.57 | 0.326 | 0.50 ± 0.68 | 0.43 ± 0.57 | −0.07 ± 0.78 | 0.645 | 0.15 ± 0.36 | 0.37 ± 0.79 | 0.22 ± 0.64 | 0.083 | 0.269 | 0.212 |
| Q13 | 0.29 ± 0.60 | 0.39 ± 0.69 | 0.11 ± 0.50 | 0.264 | 0.43 ± 0.77 | 0.43 ± 0.73 | 0.00 ± 0.98 | >0.999 | 0.30 ± 0.47 | 0.41 ± 0.75 | 0.11 ± 0.58 | 0.327 | 0.804 | 0.751 |
| Q14 | 0.32 ± 0.55 | 0.43 ± 0.79 | 0.11 ± 0.69 | 0.415 | 0.53 ± 0.86 | 0.57 ± 0.86 | 0.03 ± 0.93 | 0.845 | 0.22 ± 0.51 | 0.44 ± 0.64 | 0.22 ± 0.58 | 0.056 | 0.637 | 0.699 |
| Q15 | 0.32 ± 0.61 | 0.36 ± 0.68 | 0.04 ± 0.64 | 0.769 | 0.27 ± 0.52 | 0.33 ± 0.76 | 0.07 ± 0.87 | 0.677 | 0.26 ± 0.53 | 0.48 ± 0.75 | 0.22 ± 0.80 | 0.161 | 0.636 | 0.810 |
| Q16 | 1.04 ± 0.92 | 1.04 ± 0.96 | 0.00 ± 0.82 | >0.999 | 1.23 ± 0.94 | 1.20 ± 0.76 | −0.03 ± 1.03 | 0.861 | 0.70 ± 0.91 | 0.96 ± 0.94 | 0.26 ± 0.81 | 0.110 | 0.418 | 0.567 |
| Q17 | 0.82 ± 0.82 | 0.68 ± 0.67 | −0.14 ± 0.97 | 0.443 | 0.97± 0.89 | 0.77 ± 0.86 | −0.20 ± 0.76 | 0.161 | 0.67 ± 0.83 | 0.63 ± 0.74 | −0.04 ± 0.85 | 0.824 | 0.773 | 0.730 |
| Total score | 10.54±12.75 | 12.50 ± 13.91 | 1.96 ± 7.96 | 0.202 | 14.70 ± 12.63 | 13.13 ± 12.14 | −1.57 ± 12.71 | 0.505 | 8.00 ± 9.12 | 11.22 ± 13.69 | 3.22 ± 9.88 | 0.102 | 0.202 | 0.162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-J.; Oh, M.-R.; Lee, D.Y.; Lee, Y.-S.; Kim, G.-S.; Park, S.-H.; Han, S.-K.; Kim, Y.-O.; Yoon, S.-J.; Chae, S.-W. Effect of Ginseng Extracts on the Improvement of Osteopathic and Arthritis Symptoms in Women with Osteopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2021, 13, 3352. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103352
Jung S-J, Oh M-R, Lee DY, Lee Y-S, Kim G-S, Park S-H, Han S-K, Kim Y-O, Yoon S-J, Chae S-W. Effect of Ginseng Extracts on the Improvement of Osteopathic and Arthritis Symptoms in Women with Osteopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2021; 13(10):3352. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103352
Chicago/Turabian StyleJung, Su-Jin, Mi-Ra Oh, Dae Young Lee, Young-Seob Lee, Geum-Soog Kim, Soo-Hyun Park, Soog-Kyoung Han, Young-Ock Kim, Sun-Jung Yoon, and Soo-Wan Chae. 2021. "Effect of Ginseng Extracts on the Improvement of Osteopathic and Arthritis Symptoms in Women with Osteopenia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 13, no. 10: 3352. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103352