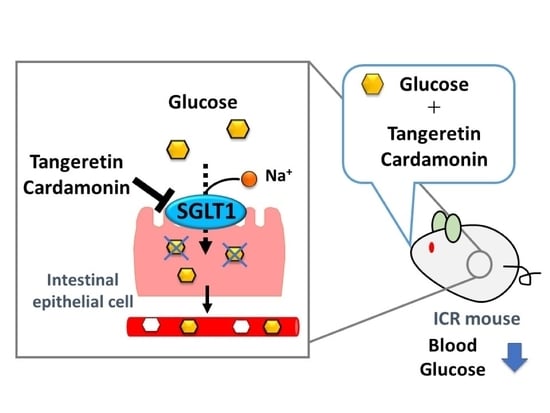
Inhibitory Effect of Tangeretin and Cardamonin on Human Intestinal SGLT1 Activity In Vitro and Blood Glucose Levels in Mice In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Transfection of Human SGLT1 Expression Vectors for Development of a Stable Cell Line
2.3. Assay of Glucose Uptake in a Stable Cell Line Expressing hSGLT1
2.4. Caco-2 Cell Culture
2.5. OGTTs in Mice
2.6. Statistical Analysis
3. Results
3.1. Construction of the Stable hSGLT1-Expressed Cell Line
3.2. Characterization of Sodium-Dependent Glucose Uptake Activity in Stable hSGLT1-Expressing Cells
3.3. Effect of Phytochemicals on Glucose Uptake in hSGLT1-Expressing CHO Cell Line
3.4. Effect of Tangeretin and Cardamonin on Nutrient Uptake in hSGLT1/CHO Cells and Human Intestinal-like Caco-2 Cells
3.5. Effect of Tangeretin-Related Compounds on Glucose Uptake in hSGLT1/CHO Cells
3.6. Effect of Cardamonin-Related Compounds on Glucose Uptake in hSGLT1/CHO Cells
3.7. Kinetic Analysis of Sodium-Dependent Glucose Uptake by hSGLT1/CHO Cells in the Absence or Presence of Tangeretin and Cardamonin
3.8. Effect of Tangeretin and Cardamonin on the Increase in Blood Glucose Levels In Vivo Following Oral Glucose Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compounds | Company |
| Myricetin | Sigma-Aldrich |
| Morin | Sigma-Aldrich |
| Puerarin | Sigma-Aldrich |
| Rutin | Wako |
| Fisetin | Sigma-Aldrich |
| Kaempherol | Extrasynthese |
| Quercitrin | Tokyo Chemical Industry |
| Quercetin | Tokyo Chemical Industry |
| Genistin | Fujicco |
| Diosmin | Sigma-Aldrich |
| Ginkgolide J | Tama Biochemical |
| Ginkgolide B | Tama Biochemical |
| Baicalein | Wako |
| Apigenin | Sigma-Aldrich |
| Flavonol | Sigma-Aldrich |
| Flavanone | Wako |
| Flavone | Sigma-Aldrich |
| Hesperetin | Sigma-Aldrich |
| Galangin | Sigma-Aldrich |
| Genistein | Sigma-Aldrich |
| Tangeretein | Wako |
| Daidzein | Sigma-Aldrich |
| Naringin | Sigma-Aldrich |
| Naringenin | Sigma-Aldrich |
| Flavone | Sigma-Aldrich |
| Daidzin | Wako |
| Equol | LC laboratories |
| Nobiletin | Wako |
| Glycitin | Wako |
| Glycitein | Wako |
| Caffeine | Sigma-Aldrich |
| Sinensetin | Wako |
| Rosmaric acid | Wako |
| Resveratrol | Wako |
| Xanthohumol | Tokyo Chemical Industry |
| 4-Hydroxyderricin | Medchemexpress |
| Xanthoangerol | Medchemexpress |
| Cardamonin | Medchemexpress |
| Isoindigo | Cayman |
| Indirubin | Tokyo Chemical Industry |
| Indigo | Wako |
| Quercetin-3,4’-di-O-Glucoside | Extrasynthese |
| Isoquercitrin | Cayman |
| Spireoside | Sigma-Aldrich |
| Chrysin | Tokyo Chemical Industry |
| Pinostrobin | Sigma-Aldrich |
| Pinocembrin | Wako |
| Alpinetin | Selleck |
| Monoglucosyl hesperidin | Wako |
| Hesperidin | Tokyo Chemical Industry |
References
- IDF. Diabetes Atlas. Available online: https://www.diabetesatlas.org/en/sections/worldwide-toll-of-diabetes.html (accessed on 12 July 2021).
- Gerich, J. Pathogenesis and management of postprandial hyperglycemia: Role of incretin-based therapies. Int. J. Gen. Med. 2013, 6, 877–895. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.B.; Hart, G.W. New insights: A role for O-GlcNAcylation in diabetic complications. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 150–161. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, R.; Shinozaki, Y.; Ohta, T. Sodium-glucose cotransporters: Functional properties and pharmaceutical potential. J. Diabetes Investig. 2020, 11, 770–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouyon, F.; Caillaud, L.; Carriere, V.; Klein, C.; Dalet, V.; Citadelle, D.; Kellett, G.L.; Thorens, B.; Leturque, A.; Brot-Laroche, E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: A study in GLUT2-null mice. J Physiol. 2003, 552, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2: A major pathway of intestinal sugar absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gromova, L.V.; Fetissov, S.O.; Gruzdkov, A.A. Mechanisms of glucose absorption in the small intestine in health and metabolic diseases and their role in appetite regulation. Nutrients 2021, 13, 2474. [Google Scholar] [CrossRef] [PubMed]
- Piasecka-Kwiatkowska, D.; Warchalewski, J.R.; Zielińska-Dawidziak, M.; Michalak, M. Digestive enzyme inhibitors from grains as potential components of nutraceuticals. J. Nutr. Sci. Vitaminol. 2012, 58, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Oishi, S.; Shudo, A.; Sugiura, Y.; Yasunaga, K. Glucose response during the night is suppressed by wheat albumin in healthy participants: A randomized controlled trial. Nutrients 2019, 11, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabbir, H.; Kausar, T.; Noreen, S.; Rehman, H.U.; Hussain, A.; Huang, Q.; Gani, A.; Su, S.; Nawaz, A. In vivo screening and antidiabetic potential of polyphenol extracts from guava pulp, seeds and leaves. Animals 2020, 10, 1714. [Google Scholar] [CrossRef]
- Satsu, H. Molecular and cellular studies on the absorption, function, and safety of food components in intestinal epithelial cells. Biosci. Biotechnol. Biochem. 2017, 81, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Satsu, H. Regulation of detoxification enzymes by food components in intestinal epithelial cells. Food Sci. Technol. Res. 2019, 25, 149–156. [Google Scholar] [CrossRef]
- Ishizuka, K.; Kanayama, A.; Satsu, H.; Miyamoto, Y.; Furihata, K.; Shimizu, M. Identification of a taurine transport inhibitory substance in sesame seeds. Biosci. Biotechnol. Biochem. 2000, 64, 1166–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizuka, K.; Miyamoto, Y.; Satsu, H.; Sato, R.; Shimizu, M. Characteristics of lysophosphatidylcholine in its inhibition of taurine uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2002, 66, 730–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satsu, H.; Awara, S.; Unno, T.; Shimizu, M. Suppressive effect of nobiletin and epicatechin gallate on fructose uptake in human intestinal epithelial Caco-2 cells. Biosci. Biotechnol. Biochem. 2018, 82, 636–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, T.; Satsu, H.; Hatsugai, Y.; Aizawa, K.; Inakuma, T.; Nagata, S.; Sakuda, S.; Nagasawa, H.; Shimizu, M. Inhibitory effect of a bitter melon extract on the P-glycoprotein activity in intestinal Caco-2 cells. Br. J. Pharmacol. 2004, 143, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Steffansen, B.; Pedersen, M.D.L.; Laghmoch, A.M.; Nielsen, C.U. SGLT1-mediated transport in Caco-2 cells is highly dependent on cell bank origin. J. Pharm. Sci. 2017, 106, 2664–2670. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, C.P.; Thevelein, J.M.; Luyten, W. Characterization of SGLT1-mediated glucose transport in Caco-2 cell monolayers, and absence of its regulation by sugar or epinephrine. Eur. J. Pharmacol. 2021, 897, 173925. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Choi, S.S.; Choi, B.K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J. Nutr. Biochem. 2013, 24, 156–162. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.S.; Ko, H.C.; Kim, S.J. Effects of sinensetin on lipid metabolism in mature 3T3-L1 adipocytes. Phytother Res. 2013, 27, 131–134. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. An overview on cardamonin. J. Med. Food 2014, 17, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Sun, C.Y.; Lu, F.R.; Shu, X.R.; Yang, D.; Chen, L.; She, X.M.; Gregg, N.M.; Guo, T.; Hu, Y. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-κB pathway in vitro. Leuk. Res. 2012, 36, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Prasad, S.; Yadav, V.R.; Aggarwal, B.B. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr. Cancer 2012, 64, 173–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, N.; Kawabata, K.; Sawada, K.; Ueda, M.; Fukuda, I.; Kawasaki, K.; Murakami, A.; Ashida, H. Cardamonin stimulates glucose uptake through translocation of glucose transporter-4 in L6 myotubes. Phytother. Res. 2011, 25, 1218–1224. [Google Scholar] [CrossRef]
- Goto, T.; Horita, M.; Nagai, H.; Nagatomo, A.; Nishida, N.; Matsuura, Y.; Nagaoka, S. Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract. Mol. Nutr. Food Res. 2012, 56, 435–445. [Google Scholar] [CrossRef]
- Yuca, H.; Özbek, H.; Demirezer, L.Ö.; Kasil, H.G.; Güvenalp, Z. Trans-tiliroside: A potent α-glucosidase inhibitor from the leaves of Elaeagnus angustifolia L. Phytochmistry 2021, 188, 112795. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Saito, K.; Yamakawa, H.; Choi, S.S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 79, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, M.S.; Woo, J.T.; Jeong, M.J.; Kim, S.R.; Jung, U.J. Long-term dietary supplementation with low-dose nobiletin ameliorates hepatic steatosis, insulin resistance, and inflammation without altering fat mass in diet-induced obesity. Mol. Nutr. Food Res. 2017, 61, 1600889. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Kawabata, K.; Sawada, K.; Ikeda, K.; Fukuda, I.; Kawasaki, K.; Yamamoto, N.; Ashida, H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol. Nutr. Food Res. 2011, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satsu, H.; Shibata, R.; Suzuki, H.; Kimura, S.; Shimizu, M. Inhibitory Effect of Tangeretin and Cardamonin on Human Intestinal SGLT1 Activity In Vitro and Blood Glucose Levels in Mice In Vivo. Nutrients 2021, 13, 3382. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103382
Satsu H, Shibata R, Suzuki H, Kimura S, Shimizu M. Inhibitory Effect of Tangeretin and Cardamonin on Human Intestinal SGLT1 Activity In Vitro and Blood Glucose Levels in Mice In Vivo. Nutrients. 2021; 13(10):3382. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103382
Chicago/Turabian StyleSatsu, Hideo, Ryosuke Shibata, Hiroto Suzuki, Shimon Kimura, and Makoto Shimizu. 2021. "Inhibitory Effect of Tangeretin and Cardamonin on Human Intestinal SGLT1 Activity In Vitro and Blood Glucose Levels in Mice In Vivo" Nutrients 13, no. 10: 3382. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13103382