Understanding the Functional Activity of Polyphenols Using Omics-Based Approaches
Abstract
:1. Introduction
2. Classification, Source and Function of Polyphenols
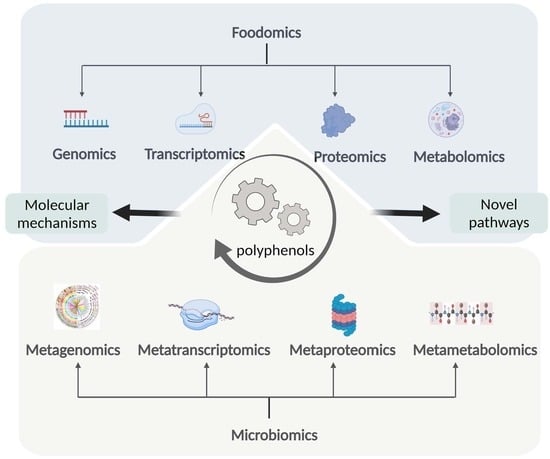
3. Foodomics Applied in the Study of Polyphenols
3.1. Genomics
3.2. Transcriptomics
3.3. Proteomics
3.4. Metabolomics
3.5. Multi-Omics
4. Microbiomics Involved in the Bioactivity of Polyphenols
4.1. Regulation of Polyphenols on Gut Microbiota
4.2. Combination of Microbiome and Metabolomics in Polyphenol Study
5. Conclusions and Further Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- An, J.; Hao, D.; Zhang, Q.; Chen, B.; Zhang, R.; Wang, Y.; Yang, H. Natural products for treatment of bone erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int. Immunopharmacol. 2016, 36, 118–131. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Wided, M.K.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. LC-MS identification and preparative HPLC isolation of Frankenia pulverulenta phenolics with antioxidant and neuroprotective capacities in PC12 cell line. Pharm. Biol. 2017, 55, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-Graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017, 61, 1600950. [Google Scholar] [CrossRef]
- Odongo, G.A.; Schlotz, N.; Herz, C.; Hanschen, F.S.; Baldermann, S.; Neugart, S.; Trierweiler, B.; Frommherz, L.; Franz, C.M.A.P.; Ngwene, B.; et al. The role of plant processing for the cancer preventive potential of Ethiopian kale (Brassica carinata). Food Nutr. Res. 2017, 61, 1271527. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, T.; Zhang, X.; Ueyama, Y.; Apisada, K.; Nakayama, M.; Suzuki, Y.; Ozawa, T.; Mitani, A.; Shigemune, N.; Shimatani, K.; et al. Development of novel monoclonal antibodies directed against catechins for investigation of antibacterial mechanism of catechins. J. Microbiol. Methods 2017, 137, 6–13. [Google Scholar] [CrossRef]
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef]
- Scicutella, F.; Mannelli, F.; Daghio, M.; Viti, C.; Buccioni, A. Polyphenols and organic acids as alternatives to antimicrobials in poultry rearing: A review. Antibiotics 2021, 10, 1010. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Effects of Botanical Polyphenol on Antioxidant Capacity, Intestinal Morphology and Meat Quality of Yellow Broilers-All Databases. Available online: https://www.webofscience.com/wos/alldb/full-record/CSCD:6423241 (accessed on 29 October 2021).
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, prunella vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from different geographic origins decreases intestinal inflammation and Bacteroides Spp. Populations in a model of DSS-induced colitis. Mol. Nutr. Food Res. 2018, 62, 1800080. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Serrano, J.; Goni, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant Capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian Australas. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Cm, O.; Ih, O.; Bk, C.; Ty, Y.; Jm, C.J.H. Consuming green tea at least twice each day is associated with reduced odds of chronic obstructive lung disease in middle-aged and older korean adults. J. Nutr. 2018, 148, 70–76. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Ren, G.; Yin, L.; Liang, X.; Geng, T.; Dang, H.; An, R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016, 1650, 1–9. [Google Scholar] [CrossRef]
- Castilla, P.; Echarri, R.; Dávalos, A.; Cerrato, F.; Ortega, H.; Teruel, J.L.; Lucas, M.F.; Gómez-Coronado, D.; Ortuño, J.; Lasunción, M.A. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutr. 2006, 84, 252–262. [Google Scholar] [CrossRef]
- Yang, F.; Xie, C.; Li, J.; Ma, R.; Dang, Z.; Wang, C.; Wang, T. Foodomics technology: Promising analytical methods of functional activities of plant polyphenols. Eur. Food Res. Technol. 2021, 247, 2129–2142. [Google Scholar] [CrossRef]
- Mondello, L. Foodomics—Advanced Mass Spectrometry in Modern Food Science and Nutrition; Cifuentes, A., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; 580p, ISBN 978-1-118-16945-2. [Google Scholar]
- Andjelkovic, U.; Gajdosik, M.S.; Gaso-Sokac, D.; Martinovic, T.; Josic, D. Foodomics and food safety: Where we are. Food Technol. Biotechnol. 2017, 55, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Fukao, H.; Ijiri, Y.; Miura, M.; Hashimoto, M.; Yamashita, T.; Fukunaga, C.; Oiwa, K.; Kawai, Y.; Suwa, M.; Yamamoto, J. Effect of trans-resveratrol on the thrombogenicity and atherogenicity in apolipoprotein e-deficient and low-density lipoprotein receptor-deficient mice. Blood Coagul. Fibrinolysis 2004, 15, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxidative Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [Green Version]
- Vallance, T.M.; Ravishankar, D.; Albadawi, D.A.I.; Osborn, H.M.I.; Vaiyapuri, S. Synthetic flavonoids as novel modulators of platelet function and thrombosis. IJMS 2019, 20, 3106. [Google Scholar] [CrossRef] [Green Version]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa Ștefan, C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Identification of lignans as major components in the phenolic fraction of olive oil. Clin. Chem. 2000, 46, 976–988. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Ito, H. Tannins of Constant structure in medicinal and food plants-hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Kingston-Smith, A.H.; Winters, A.L.; Lee, M.R.F.; Minchin, F.R.; Morris, P.; MacRae, J. Polyphenols and their influence on gut function and health in ruminants: A review. Environ. Chem. Lett. 2006, 4, 121–126. [Google Scholar] [CrossRef]
- Geng, D.; Fang, M.Y.; Deli, L.I.; Zheng, S.Q.; Lijun, D.U. Research progress in terms of interaction between chinese medicine components and intestinal microenvironment. Sci. Sin. 2018, 48, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [Green Version]
- De Camargo, A.C.; Regitano-d’Arce, M.A.B.; Rasera, G.B.; Canniatti-Brazaca, S.G.; do Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The neuroprotective effects of phenolic acids: Molecular mechanism of action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.K.; Luo, J.-Y.; Lau, C.W.; Chen, Z.-Y.; Tian, X.Y.; Huang, Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020, 177, 1258–1277. [Google Scholar] [CrossRef]
- Varoni, E.M.; lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An interplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and their derivatives from plants as antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaheer, K.; Akhtar, M.H. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef] [Green Version]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Kilani-Jaziri, S.; Mustapha, N.; Mokdad-Bzeouich, I.; El Gueder, D.; Ghedira, K.; Ghedira-Chekir, L. Flavones induce immunomodulatory and anti-inflammatory effects by activating cellular anti-oxidant activity: A structure-activity relationship study. Tumor Biol. 2016, 37, 6571–6579. [Google Scholar] [CrossRef]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Lagana, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Butelli, E.; de Santis, S.; Cavalcanti, E.; Hill, L.; de Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined dietary anthocyanins, flavonols, and stilbenoids alleviate inflammatory bowel disease symptoms in mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Mena, P.; Dolores, G.; Brindani, N.; Esteban-Fernández, A.; Curti, C.; Moreno-Arribas, M.V.; Rio, D.D.; Bartolomé, B. 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone and its sulphate conjugates, representative circulating metabolites of flavan-3-ols, exhibit anti-adhesive activity against uropathogenic escherichia coli in bladder epithelial cells. J. Funct. Foods 2017, 29, 275–280. [Google Scholar] [CrossRef]
- Fayeulle, N.; Vallverdu-Queralt, A.; Meudec, E.; Hue, C.; Boulanger, R.; Cheynier, V.; Sommerer, N. Characterization of new flavan-3-Ol derivatives in fermented cocoa beans. Food Chem. 2018, 259, 207–212. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.M.; Almagribi, W.; Al-Rashidi, M.N. Antiradical and reductant activities of anthocyanidins and anthocyanins, structure-activity relationship and synthesis. Food Chem. 2016, 194, 1275–1282. [Google Scholar] [CrossRef]
- Mudd, A.M.; Gu, T.; Munagala, R.; Jeyabalan, J.; Egilmez, N.K.; Gupta, R.C. Chemoprevention of colorectal cancer by anthocyanidins and mitigation of metabolic shifts induced by dysbiosis of the gut microbiome. Cancer Prev. Res. 2020, 13, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Lin, Y.-M.; Zhou, H.-C.; Wei, S.-D.; Chen, J.-H. Condensed tannins from mangrove species kandelia candel and rhizophora mangle and their antioxidant activity. Molecules 2010, 15, 420–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects: Pharmacological aspects of tannins. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tie, F.; Wang, J.; Liang, Y.; Zhu, S.; Wang, Z.; Li, G.; Wang, H. Proanthocyanidins ameliorated deficits of lipid metabolism in type 2 diabetes mellitus via inhibiting adipogenesis and improving mitochondrial function. IJMS 2020, 21, 2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, T.; Calcabrini, C.; Diaz, A.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Rasines-Perea, Z.; Jacquet, R.; Jourdes, M.; Quideau, S.; Teissedre, P.-L. Ellagitannins and flavano-ellagitannins: Red wines tendency in different areas, barrel origin and ageing time in barrel and bottle. Biomolecules 2019, 9, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Y.; Gao, G.; Chen, W. Research Progress of concepts and techniques related to post-genome era. J. Suzhou Coll. 2007, 4, 101–103. [Google Scholar]
- Chen, J.; Uto, T.; Tanigawa, S.; Kumamoto, T.; Fujii, M.; Hou, D.-X. Expression profiling of genes targeted by bilberry (Vaccinium myrtillus) in macrophages through DNA microarray. Nutr. Cancer 2008, 60, 43–50. [Google Scholar] [CrossRef]
- Chen, J.; Qin, S.; Xiao, J.; Tanigawa, S.; Uto, T.; Hashimoto, F.; Fujii, M.; Hou, D.X. A Genome-wide microarray highlights the antiinflammatory genes targeted by oolong tea theasinensin a in macrophages. Nutr. Cancer 2011, 63, 1064–1073. [Google Scholar] [CrossRef]
- Verdu, C.F.; Guyot, S.; Childebrand, N.; Bahut, M.; Celton, J.-M.; Gaillard, S.; Lasserre-Zuber, P.; Troggio, M.; Guilet, D.; Laurens, F. QTL analysis and candidate gene mapping for the polyphenol content in cider apple. PLoS ONE 2014, 9, e107103. [Google Scholar] [CrossRef] [Green Version]
- Ding, T. The Evaluation of Polyphenol in Peach and Its QTL Mapping. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2017. [Google Scholar]
- Tian, J. Genome Mining for the Pentangular Polyphenols. Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 2016. [Google Scholar]
- Hou, D.-X.; Luo, D.; Tanigawa, S.; Hashimoto, F.; Uto, T.; Masuzaki, S.; Fujii, M.; Sakata, Y. Prodelphinidin B-4 3′-O-gallate, a tea polyphenol, is involved in the inhibition of COX-2 and INOS via the downregulation of TAK1-NF-KappaB pathway. Biochem. Pharmacol. 2007, 74, 742–751. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Gu, Z.; Wang, Y.; Shi, M.; Ji, Y.; Sun, J.; Xu, X.; Zhang, L.; Jiang, J.; et al. Resveratrol inhibits enterovirus 71 replication and pro-inflammatory cytokine secretion in rhabdosarcoma cells through blocking IKKs/NF-ΚB signaling pathway. PLoS ONE 2015, 10, e0116879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harasstani, O.A.; Moin, S.; Tham, C.L.; Liew, C.Y.; Ismail, N.; Rajajendram, R.; Harith, H.H.; Zakaria, Z.A.; Mohamad, A.S.; Sulaiman, M.R. Flavonoid combinations cause synergistic inhibition of proinflammatory mediator secretion from lipopolysaccharide-induced RAW 264.7 cells. Inflamm. Res. 2010, 59, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPbeta DNA-binding activity. Immunobiology 2006, 211, 359–368. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, I.J.; Lee, H.-Y.; Han, S.-B.; Hong, J.T.; Ahn, B.; Lee, C.-K.; Kim, Y. Quercetin 3-O-beta-(2”-galloyl)-glucopyranoside inhibits endotoxin LPS-induced IL-6 expression and NF-KB activation in macrophages. Cytokine 2007, 39, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, S.; Fujii, M.; Hou, D.-X. Action of Nrf2 and keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
- Hou, D.X.; Fukuda, M.; Johnson, J.A.; Miyamori, K.; Ushikai, M.; Fujii, M. Fisetin induces transcription of NADPH: Quinone oxidoreductase gene through an antioxidant responsive element-involved activation. Int. J. Oncol. 2001, 18, 1175–1179. [Google Scholar] [PubMed]
- Hou, D.X.; Fukuda, M.; Fujii, M.; Fuke, Y. Transcriptional regulation of nicotinamide adenine dinucleotide phosphate: Quinone oxidoreductase in murine hepatoma cells by 6-(methylsufinyl)hexyl isothiocyanate, an active principle of wasabi (Eutrema wasabi Maxim). Cancer Lett. 2000, 161, 195–200. [Google Scholar] [CrossRef]
- Altamemi, I.; Murphy, E.A.; Catroppo, J.F.; Zumbrun, E.E.; Zhang, J.; McClellan, J.L.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Role of MicroRNAs in resveratrol-mediated mitigation of colitis-associated tumorigenesis in Apc(Min/+) mice. J. Pharmacol. Exp. Ther. 2014, 350, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Guodong, R.; Jianguo, Z.; Xiaoxia, L.; Ying, L. Identification of putative genes for polyphenol biosynthesis in olive fruits and leaves using full-length transcriptome sequencing. Food Chem. 2019, 300, 125246. [Google Scholar] [CrossRef]
- Valdés, A.; Sullini, G.; Ibáñez, E.; Cifuentes, A.; García-Cañas, V. Rosemary polyphenols induce unfolded protein response and changes in cholesterol metabolism in colon cancer cells. J. Funct. Foods 2015, 15, 429–439. [Google Scholar] [CrossRef]
- Figeys, D.; Pinto, D. Proteomics on a chip: Promising developments. Electrophoresis 2001, 22, 208–216. [Google Scholar] [CrossRef]
- Díaz-Chávez, J.; Fonseca-Sánchez, M.A.; Arechaga-Ocampo, E.; Flores-Pérez, A.; Palacios-Rodríguez, Y.; Domínguez-Gómez, G.; Marchat, L.A.; Fuentes-Mera, L.; Mendoza-Hernández, G.; Gariglio, P.; et al. Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS ONE 2013, 8, e64378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.-J.; Chen, X.-W.; Wang, J.-Z.; Ju, Y.-L.; Yang, M.-Z.O.; Zhang, W.-J. Proteomic analysis identifies proteins associated with curcumin-enhancing efficacy of irinotecan-induced apoptosis of colorectal cancer LOVO cell. Int. J. Clin. Exp. Pathol. 2014, 7, 1–15. [Google Scholar]
- Xu, R.; Tian, E.; Tang, H.; Liu, C.; Wang, Q. Proteomic analysis of gossypol induces necrosis in multiple myeloma cells. BioMed. Res. Int. 2014, 2014, 839232. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Huang, J.; Liang, Q.; Yan, Y.; Lin, H.; Xiao, W.; Lin, Y.; Zhang, S.; Tan, B.; et al. Proteomic analysis of the inhibitory effect of epigallocatechin gallate on lipid accumulation in human HepG2 cells. Proteome Sci. 2013, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Liang, S.; Fang, L.; Chen, L.; Tang, M.; Xu, Y.; Fu, A.; Yang, J.; Wei, Y. Quantitative proteomic analysis of HepG2 cells treated with quercetin suggests IQGAP1 involved in quercetin-induced regulation of cell proliferation and migration. OMICS 2009, 13, 93–103. [Google Scholar] [CrossRef]
- Oliver, S. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef]
- Liu, P.; Wang, P. Application of metabolomics technology in the research of Chinese medicine. Chin. J. Integr. Med. 2014, 20, 307–310. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Shen, C.-L.; Wang, J.-S. Green tea polyphenols modify gut-microbiota dependent metabolisms of energy, bile constituents and micronutrients in female sprague-dawley rats. J. Nutr. Biochem. 2018, 61, 68–81. [Google Scholar] [CrossRef]
- Szekeres, T.; Saiko, P.; Fritzer-Szekeres, M.; Djavan, B.; Jäger, W. Chemopreventive effects of resveratrol and resveratrol derivatives: Chemopreventive effects of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Tsoy, I.; Park, J.M.; Chung, J.I.; Shin, S.C.; Chang, K.C. Anthocyanins from soybean seed coat inhibit the expression of TNF-α-induced genes associated with ischemia/reperfusion in endothelial cell by NF-ΚB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006, 580, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X. Effects of Tea Polyphenols EGCG on Gut Microbiota and Metabolism in Mice Based on Bioinformatics. Master’s Thesis, Nanchang University, Nanchang, China, 2019. [Google Scholar]
- Li, Q.; Liang, X.; Guo, N.; Hu, L.; Prasad, M.E.; Wu, Y.; Xue, X.; Wu, L.; Wang, K. Protective effects of bee pollen extract on the caco-2 intestinal barrier dysfunctions induced by dextran sulfate sodium. Biomed. Pharmacother. 2019, 117, 109200. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Stability of dietary polyphenols: It’s never too late to mend? Food Chem. Toxicol. 2018, 119, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMS(n.). Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Characterization of phenolic compounds and antioxidant activity of solanum scabrum and solanum burbankii berries. J. Agric. Food Chem. 2014, 62, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Foglia, P.; Gubbiotti, R.; Sacchetti, P.; Samperi, R.; Laganà, A. Rapid-resolution liquid chromatography/mass spectrometry for determination and quantitation of polyphenols in grape berries. Rapid Commun. Mass Spectrom. 2008, 22, 3089–3099. [Google Scholar] [CrossRef]
- Ma, S.; Kim, C.; Neilson, A.P.; Griffin, L.E.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Comparison of common analytical methods for the quantification of total polyphenols and flavanols in fruit juices and ciders. J. Food Sci. 2019, 84, 2147–2158. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.B.; Lebelle, J.; Birsan, R.; Rai, D.K. Enrichment and assessment of the contributions of the major polyphenols to the total antioxidant activity of onion extracts: A fractionation by flash chromatography approach. Antioxidants 2018, 7, E175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. Convar. Acephala Var. Sabellica) by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2009, 57, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Llorach, R.; Gil-Izquierdo, A.; Ferreres, F.; Tomás-Barberán, F.A. HPLC-DAD-MS/MS ESI characterization of unusual highly glycosylated acylated flavonoids from cauliflower (Brassica oleracea L. Var. Botrytis) agroindustrial byproducts. J. Agric. Food Chem. 2003, 51, 3895–3899. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Lee, M.-H.; Park, C.-H.; Pae, S.-B.; Shim, K.-B.; Ko, J.-M.; Shin, S.-O.; Baek, I.-Y.; Park, K.-Y. Identification and characterization of anthocyanins in yard-long beans (Vigna unguiculata ssp. Sesquipedalis L.) by high-performance liquid chromatography with diode array detection and electrospray ionization/mass spectrometry (HPLC-DAD-ESI/MS) analysis. J. Agric. Food Chem. 2010, 58, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.; Blanchard, C. Q-TOF LC/MS identification and UHPLC-online ABTS antioxidant activity guided mapping of barley polyphenols. Food Chem. 2018, 266, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C. Quantification of almond skin polyphenols by liquid chromatography-mass spectrometry. J. Food Sci. 2009, 74, C326–C332. [Google Scholar] [CrossRef]
- González-Barrio, R.; Nuñez-Gomez, V.; Cienfuegos-Jovellanos, E.; García-Alonso, F.J.; Periago-Castón, M.J. Improvement of the flavanol profile and the antioxidant capacity of chocolate using a phenolic rich cocoa powder. Foods 2020, 9, E189. [Google Scholar] [CrossRef] [Green Version]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Zhuang, J.; Dai, X.; Zhu, M.; Zhang, S.; Dai, Q.; Jiang, X.; Liu, Y.; Gao, L.; Xia, T. Evaluation of astringent taste of green tea through mass spectrometry-based targeted metabolic profiling of polyphenols. Food Chem. 2020, 305, 125507. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalan, J.; Medina-Remon, A.; Sasot, G.; Mercader-Martí, M.; Llobet, J.M.; Lamuela-Raventos, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-orbitrap-MS. Food Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Wang, L.; Zhang, Y.; Ruan, L.; Li, H.; Wu, L.; Xu, L.; Zhang, C.; Zhou, X.; Cheng, H.; et al. A coupled role for CsMYB75 and CsGSTF1 in anthocyanin hyperaccumulation in purple tea. Plant J. 2019, 97, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Jiang, X.; Dai, X.; Xia, T. Evolutionary and functional characterization of leucoanthocyanidin reductases from Camellia sinensis. Planta 2018, 247, 139–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef]
- Zhang, Y.; Owusu, L.; Duan, W.; Jiang, T.; Zang, S.; Ahmed, A.; Xin, Y. Anti-metastatic and differential effects on protein expression of epigallocatechin-3-gallate in HCCLM6 hepatocellular carcinoma cells. Int. J. Mol. Med. 2013, 32, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Ménoret, A.; Drew, D.A.; Miyamoto, S.; Nakanishi, M.; Vella, A.T.; Rosenberg, D.W. Differential proteomics identifies PDIA3 as a novel chemoprevention target in human colon cancer cells. Mol. Carcinog. 2014, 53, E11–E22. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [Green Version]
- Larrosa, M.; Yanez-Gascon, J.M.; Selma, V.M.; Gonzalez-Sarrias, A.; Toti, S.; Ceron, J.J.; Tomas-Barberan, F.; Dolara, P.; Espin, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Kim, N.; Kunisawa, J.; Kweon, M.-N.; Ji, G.E.; Kiyono, H. Oral feeding of Bifidobacterium bifidum (BGN4) prevents CD4(+) CD45RB(High) T cell-mediated inflammatory bowel disease by inhibition of disordered T cell activation. Clin. Immunol. 2007, 123, 30–39. [Google Scholar] [CrossRef]
- Yuan, X.; Long, Y.; Ji, Z.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X.; et al. Green tea liquid consumption alters the human intestinal and oral microbiome. Mol. Nutr. Food Res. 2018, 62, 1800178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Hu, M.; Zhang, L.; Gao, Y.; Ma, L.; Xu, Q. Dietary taxifolin protects against dextran sulfate sodium-induced colitis via NF-ΚB signaling, enhancing intestinal barrier and modulating gut microbiota. Front. Immunol. 2020, 11, 631809. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, Y.; Chu, Q.; Zhang, P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019, 447, 41–47. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- De Bruyne, T.; Steenput, B.; Roth, L.; de Meyer, G.R.Y.; Santos, C.N.D.; Valentová, K.; Dambrova, M.; Hermans, N. Dietary Polyphenols targeting arterial stiffness: Interplay of contributing mechanisms and gut microbiome-related metabolism. Nutrients 2019, 11, E578. [Google Scholar] [CrossRef] [Green Version]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa polyphenols and gut microbiota interplay: Bioavailability, prebiotic effect, and impact on human health. Nutrients 2020, 12, E1908. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M. Pomegranate protection against cardiovascular diseases. Evid. Based Complement. Alternat. Med. 2012, 2012, 382763. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, L.; Shen, C.-L.; Wang, J.-S. Green tea polyphenols boost gut-microbiota-dependent mitochondrial TCA and urea cycles in Sprague-Dawley rats. J. Nutr. Biochem. 2020, 81, 108395. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shan, S.; Zhang, C.; Shi, J.; Li, H.; Li, Z. Inhibitory effects of bound polyphenol from foxtail millet bran on colitis-associated carcinogenesis by the restoration of gut microbiota in a mice model. J. Agric. Food Chem. 2020, 68, 3506–3517. [Google Scholar] [CrossRef] [PubMed]




| Polyphenols | Subclass | Sources | Function | Ref. |
|---|---|---|---|---|
| Phenolic acids | Coffee, berries, kiwi, apple, cherry | Anti-inflammatory, anti-oxidant, antibacterial, antiviral, antiparasitic | [38,39,40] | |
| Stilbenes | Grapes, wine | Anti-inflammatory, anti-oxidant, heart protection, anti-cancer, anti-obesity | [41,42,43] | |
| Lignans | Linseed, sesame, wheat | Anti-tumor, scavenging free radicals, anti-oxidant | [44,45,46] | |
| Flavonoids | ||||
| Isoflavones | Soy, miso | Estrogenic activity, anti-inflammatory, anti-obesity, anti-diabetic, anti-oxidant, cholesterol lowering | [47,48,49] | |
| Flavones | Parsley, celery, capsicum pepper | Anti-inflammatory, anti-oxidant, regulating glucose and lipid metabolism, anti-virus, anti-bacterial, anti-parasitic | [50,51,52] | |
| Flavanones | Grapefruit, lemon, oranges | Anti-inflammatory, anti-oxidant, regulating glucose and lipid metabolism, preventing liver steatosis, anti-bacterial, anti-viral, anti-parasitic, anti-fungal | [53,54] | |
| Flavonols | Berries, onion, broccoli, leek | Anti-inflammatory, anti-oxidant, anti-virus, anti-bacterial | [55,56,57] | |
| Flavanols | Grapes, cocoa, wine, apricots, green tea, beans | Anti-inflammatory, anti-oxidant, antibacterial, antiviral, antiparasitic, anticancer | [58,59,60] | |
| Anthocyanins | Berries, black grapes, aubergine, red wine, rhubarb | Anti-inflammatory, anti-bacterial, anti-oxidant, anti-diabetic, anti-cancer, nerve protection, anti-allergic | [61,62,63] | |
| Tannins | Condensed tannins | Cocoa, chocolate, apples, grapes | Anti-oxidant, eliminating free radicals, enhancing immunity, preventing cardiovascular and cerebrovascular diseases, improving hypoxia | [64,65,66] |
| Hydrolyzable tannins | Mango, pomegranate | Anti-oxidant, anticancer, phytoestrogens activity | [67,68] |
| Polyphenol-Rich Foods | Polyphenols Identification | MS Based Tools | Ref. |
|---|---|---|---|
| Strawberry | Cyanidin, ellagic acid derivatives, glycosides of quercetin, kaempferol, taxifolin 3-O-arabinoside, peonidin, pelargonidin | UHPLC-HR-MS | [100] |
| Blueberry | Anthocyanins, flavonols, flavan-3-ols, resveratrol, phenolic acids | HPLC-IT-TOF-MS | [101] |
| Cherry | Hydroxycinnamic acids, anthocyanins, flavonoids | LC-ESI-Q-TOF-MS | [102] |
| Berry | Delphinidin-3-O-rutinoside-5-O-glucoside; 5-caffeoylquinic acid, quercetin-3-O-rutinoside, quercetin-3-O-glucoside, petunidin-3-O-rutinoside-5-O-glucoside, 3-caffeoylquinic acid, malvidin-3-O-rutinoside-5-O-glucoside, 4-caffeoylquinic acid | UPLC-PDA-Q-TOF-MS | [103] |
| Grape | Anthocyanins, flavan-3-ols, flavonols, stilbenes | LC-MS | [104] |
| Apples | Procyanidin, chlorogenic acid, quercetin, (+)-catechin, (−)-epicatechin | UPLC/MS | [105] |
| Onions | Quercetin-4′-glucoside, quercetin-3,4′-diglucoside | LC-MS/MS | [106] |
| Cabbage | Quercetin-3-disinapoyl-triglucoside-7-diglucoside, kaempferol 3-di(tri, feruloyldi, sinapoyltri, disinapoyltri)glucoside-7-diglucoside | HPLC-DAD-ESI-MS, HPLC-DAD-MS | [107,108] |
| Beans | Delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, cyanidin-3-O-sambubioside, pelargonidin-3-O-glucoside, malvidin-3-O-glucoside, cyanidin-3-O-galactoside, petunidin-3-O-glucoside | HPLC-ESI-MS | [109] |
| Barley | Caffeic acid, catechin, cereals, ellagic acid, ferulic acid, gallic acid, isoscoparin-2″-O-glucoside, p-coumaric acid, procyanidin B2 | Q-TOF-LC-MS | [110] |
| Nut | (+)-catechin, (−)-epicatechin, procatechuic acid, p-hydroxybenzoic acid, quercetin-3-O-rutinoside, naringenin-7-O-glucoside | LC-MS | [111] |
| Cocoa beans | Flavan-3-ols, procyanidins, (+)-catechin, (−)-epicatechin | HPLC-DAD, HPLC-FL | [112] |
| Coffee | Phenolic acids, flavonoids, secoiridoids | HPLC-MS/MS | [113] |
| Green tea | Quercetin-3-O-galactoside, chlorogenic acid, epicatechin, epigallocatechin gallate, proanthocyanidin B2, quercetin-3-O-galactoside | LC-MS | [114] |
| Wine | Gallic acid, gentisic acid, protocatechuic acid-O-hexoside, protocatechuic acid, caftaric acid, catechin, coumaric-O-hexoside, p-hydroxybenzoic acid, caffeic acid | HPLC/ESI-LTQ-Orbitrap-MS | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, W.; Zhang, Y.; Li, X.; Du, Y.; Xu, Q. Understanding the Functional Activity of Polyphenols Using Omics-Based Approaches. Nutrients 2021, 13, 3953. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13113953
Si W, Zhang Y, Li X, Du Y, Xu Q. Understanding the Functional Activity of Polyphenols Using Omics-Based Approaches. Nutrients. 2021; 13(11):3953. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13113953
Chicago/Turabian StyleSi, Wenjin, Yangdong Zhang, Xiang Li, Yufeng Du, and Qingbiao Xu. 2021. "Understanding the Functional Activity of Polyphenols Using Omics-Based Approaches" Nutrients 13, no. 11: 3953. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13113953