Effect of Monthly Vitamin D Supplementation on Preventing Exacerbations of Asthma or Chronic Obstructive Pulmonary Disease in Older Adults: Post Hoc Analysis of a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
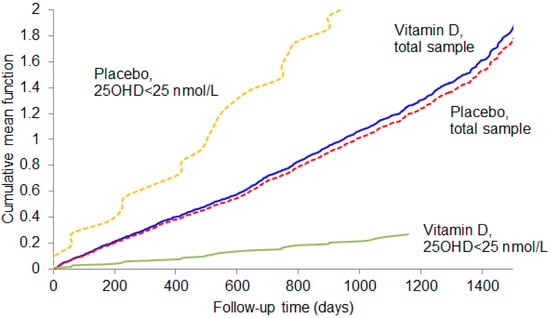
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshabanat, A.; Zafari, Z.; Albanyan, O.; Dairi, M.; Fitzgerald, J.M. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PLoS ONE 2015, 10, e0136065. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, A.I.; Farne, H.; Singanayagam, A.; Jackson, D.J.; Mallia, P.; Johnston, S.L. Pathogenesis of Viral Infection in Exacerbations of Airway Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 2), S115-32. [Google Scholar] [CrossRef]
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Available online: http://www.ginasthma.com/ (accessed on 30 October 2020).
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD. Available online: http://www.goldcopd.com/ (accessed on 30 October 2020).
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, C.A., Jr. Vitamin D, acute respiratory infection, and asthma/chronic obstructive pulmonary disease. In Vitamin D, 4th ed.; Feldman, D., Pike, J.W., Bouillon, R., Giovannucci, E., Goltzman, D., Hewison, M., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2018; pp. 1096–1120. [Google Scholar]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D supplementation to prevent asthma exacerbations: A systematic review and meta-analysis of individual participant data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; De Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 2019, 74, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; Van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2012, 156, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Martineau, A.R.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.M.; et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- Rafiq, R.; Prins, H.J.; Boersma, W.G.; Daniels, J.M.; Heijer, M.D.; Lips, P.; De Jongh, R.T. Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: A pilot trial. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2583–2592. [Google Scholar] [CrossRef] [Green Version]
- Zendehdel, A.; Gholami, M.R.; Anbari, K.; Ghanadi, K.; Bachari, E.C.; Azargoon, A. Effects of Vitamin D Intake on FEV1 and COPD Exacerbation: A Randomized Clinical Trial Study. Glob. J. Heal. Sci. 2014, 7, 243–248. [Google Scholar] [CrossRef] [Green Version]
- SScragg, R.; Waayer, D.; Stewart, A.W.; Lawes, C.M.; Toop, L.; Murphy, J.; Khaw, K.-T.; Camarago, C.A., Jr. The Vitamin D Assessment (ViDA) Study: Design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J. Steroid Biochem. Mol. Biol. 2016, 164, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.-T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaw, K.-T.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Camargo, C.A.; Scragg, R. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: Secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017, 5, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Camargo, C.A.; Sluyter, J.; Stewart, A.W.; Khaw, K.-T.; Lawes, C.M.M.; Toop, L.; Waayer, D.; Scragg, R. Effect of Monthly High-Dose Vitamin D Supplementation on Acute Respiratory Infections in Older Adults: A Randomized Controlled Trial. Clin. Infect. Dis. 2019, 71, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Sachs, M.C.; Shoben, A.; Levin, G.P.; Robinson-Cohen, C.; Hoofnagle, A.N.; Swords-Jenny, N.; Ix, J.H.; Budoff, M.; Lutsey, P.L.; Siscovick, D.S.; et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2013, 97, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.; Goldstein, R.S.; Guyatt, G.H.; Blouin, M.; Tan, W.C.; Davis, L.L.; Heels-Ansdell, D.M.; Erak, M.; Bragaglia, P.J.; Tamari, I.E.; et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. Can. Med Assoc. J. 2010, 182, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Martinez, C.H.; Mannino, D.M.; Jaimes, F.A.; Curtis, J.L.; Han, M.K.; Hansel, N.N.; Diaz, A.A. Undiagnosed Obstructive Lung Disease in the United States. Associated Factors and Long-term Mortality. Ann. Am. Thorac. Soc. 2015, 12, 1788–1795. [Google Scholar] [CrossRef] [Green Version]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malihi, Z.; Lawes, C.M.; Wu, Z.; Huang, Y.; Waayer, D.; Toop, L.; Khaw, K.-T.; Camargo, C.A.; Scragg, R. Monthly high-dose vitamin D3 supplementation and self-reported adverse events in a 4-year randomized controlled trial. Clin. Nutr. 2019, 38, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Malihi, Z.; Lawes, C.M.M.; Wu, Z.; Huang, Y.; Waayer, D.; Toop, L.; Khaw, K.-T.; Camargo, C.A.; Scragg, R. Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: Results from a randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, C.A., Jr.; Martineau, A.R. Vitamin D to prevent COVID-19: Recommendations for the design of clinical trials. FEBS J. 2020, 287, 3689–3692. [Google Scholar] [CrossRef] [PubMed]



| Variable | Vitamin D (n = 402) | Placebo (n = 373) | p-Value |
|---|---|---|---|
| Age (years) | 0.77 | ||
| 50–59 | 84 (20.9) | 75 (20.1) | |
| 60–69 | 169 (42.0) | 152 (40.8) | |
| 70–79 | 128 (31.8) | 120 (32.2) | |
| 80–84 | 21 (5.2) | 26 (7.0) | |
| Sex–male | 227 (56.5) | 210 (56.3) | 0.96 |
| Ethnicity | 0.25 | ||
| Māori | 30 (7.5) | 38 (10.2) | |
| Pacific Island | 15 (3.7) | 22 (5.9) | |
| South Asian | 16 (4.0) | 13 (3.5) | |
| European/Other | 341 (84.8) | 300 (80.4) | |
| Education (highest level) a | 0.71 | ||
| Primary school | 8 (2.0) | 5 (1.3) | |
| Secondary school | 198 (49.3) | 179 (48.0) | |
| Tertiary | 196 (48.8) | 189 (50.7) | |
| In paid employment a | 0.45 | ||
| Yes | 203 (50.5) | 174 (46.8) | |
| No | |||
| Retired | 172 (42.8) | 166 (44.6) | |
| Other | 27 (6.7) | 32 (8.6) | |
| Tobacco smoking | 0.95 | ||
| Current | 50 (12.4) | 46 (12.3) | |
| Ex | 250 (62.2) | 236 (63.3) | |
| Never | 102 (25.4) | 91 (24.4) | |
| Vigorous physical activity (hours per week) a | 0.09 | ||
| None | 149 (38.6) | 163 (46.4) | |
| 1–2 | 102 (26.4) | 76 (21.7) | |
| >2 | 135 (35.0) | 112 (31.9) | |
| Take vitamin D supplements b | 25 (6.2) | 31 (8.3) | 0.26 |
| Type of asthma/COPD | 0.07 | ||
| Asthma only | 106 (26.4) | 108 (29.0) | |
| COPD only | 200 (49.8) | 156 (41.8) | |
| Combined asthma & COPD | 96 (23.9) | 109 (29.2) | |
| Spirometry, mean (SD) | |||
| FEV1 (L) | 2.18 (0.71) | 2.15 (0.70) | 0.53 |
| FEV1, % predicted | 78 (19) | 79 (19) | 0.98 |
| FVC (L) | 3.27 (0.98) | 3.21 (0.93) | 0.37 |
| FVC, % predicted | 90 (17) | 91 (17) | 0.79 |
| Ratio FEV1/FVC | 0.67 (0.09) | 0.67 (0.10) | 0.69 |
| FEV1/FVC, % predicted | 86 (12) | 86 (11) | 0.70 |
| PEF (L/min) | 353 (116) | 360 (118) | 0.43 |
| Anthropometry, mean (SD) | |||
| Height (cm) | 168.9 (9.3) | 168.6 (9.2) | 0.70 |
| Weight (kg) | 80.3 (16.2) | 80.6 (17.3) | 0.81 |
| Body mass index (kg/m2) | 28.1 (5.1) | 28.3 (5.6) | 0.57 |
| Corrected serum calcium, mean (SD) (mmol/L) | 2.28 (0.07) | 2.27 (0.07) | 0.10 |
| 25-hydroxyvitamin D (nmol/L) | |||
| Observed, mean (SD) | 64.5 (23.1) | 60.4 (24.2) | 0.02 |
| Deseasonalized, mean (SD) | 66.7 (22.3) | 63.1 (22.9) | 0.03 |
| Deseasonalized category | 0.03 | ||
| <25.0 | 8 (2.0) | 10 (2.7) | |
| 25.0–49.9 | 82 (20.4) | 109 (29.2) | |
| 50.0–74.9 | 172 (42.8) | 134 (35.9) | |
| ≥75.0 | 140 (34.8) | 120 (32.2) |
| Participants | Vitamin D | Placebo | Hazard Ratio (95%CI) a | p-Value (Wald X2) | ||||
|---|---|---|---|---|---|---|---|---|
| Number of Participants | Number of Prescriptions /p-Y | Incidence Rate per p-Y | Number of Participants | Number of Prescriptions /p-Y | Incidence Rate per p-Y | |||
| All | 402 | 536/1324 | 0.40 | 373 | 469/1218 | 0.39 | 1.08 (0.84, 1.39) | 0.55 |
| Asthma-COPD category | ||||||||
| Asthma only | 106 | 172/352 | 0.49 | 108 | 173/367 | 0.47 | 1.18 (0.80, 1.74) | 0.41 |
| COPD only | 200 | 176/650 | 0.27 | 156 | 124/494 | 0.25 | 1.07 (0.69, 1.67) | 0.75 |
| Combined asthma & COPD | 96 | 188/320 | 0.59 | 109 | 172/357 | 0.48 | 1.21 (0.78, 1.88) | 0.39 |
| Age (years) | ||||||||
| 50–59 | 84 | 155/299 | 0.52 | 75 | 117/270 | 0.43 | 1.23 (0.73, 2.07) | 0.43 |
| 60–69 | 169 | 220/550 | 0.40 | 152 | 206/494 | 0.42 | 0.99 (0.68, 1.45) | 0.97 |
| 70–79 | 128 | 139/410 | 0.34 | 120 | 108/374 | 0.29 | 1.21 (0.73, 2.03) | 0.46 |
| 80–84 | 21 | 22/65 | 0.34 | 26 | 38/80 | 0.48 | 0.54 (0.19, 1.52) | 0.24 |
| Sex | ||||||||
| Male | 227 | 242/744 | 0.33 | 210 | 270/676 | 0.40 | 0.83 (0.58, 1.18) | 0.30 |
| Female | 175 | 294/580 | 0.51 | 163 | 199/542 | 0.37 | 1.46 (1.03, 2.06) | 0.03 |
| Ethnicity | ||||||||
| Māori | 30 | 41/100 | 0.41 | 38 | 72/127 | 0.57 | 0.68 (0.33, 1.42) | 0.31 |
| Pacific Island | 15 | 48/53 | 0.91 | 22 | 49/73 | 0.67 | 1.37 (0.56, 3.31) | 0.49 |
| South Asian | 16 | 33/51 | 0.65 | 13 | 20/43 | 0.47 | 1.73 (0.51, 5.92) | 0.38 |
| European/Other | 341 | 414/1120 | 0.37 | 300 | 328/976 | 0.34 | 1.10 (0.82, 1.46) | 0.54 |
| 25OHD category (nmol/L) b | ||||||||
| <25.0 | 8 | 2/27 | 0.07 | 10 | 23/33 | 0.70 | 0.11 (0.02, 0.51) | 0.005 |
| 25.0–49.9 | 82 | 152/265 | 0.57 | 109 | 150/360 | 0.42 | 1.45 (0.92, 2.30) | 0.11 |
| 50.0–74.9 | 172 | 232/569 | 0.41 | 134 | 168/437 | 0.38 | 1.09 (0.76, 1.58) | 0.64 |
| ≥75.0 | 140 | 150/463 | 0.32 | 120 | 128/389 | 0.33 | 1.03 (0.62, 1.70) | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, C.A., Jr.; Toop, L.; Sluyter, J.; Lawes, C.M.M.; Waayer, D.; Khaw, K.-T.; Martineau, A.R.; Scragg, R. Effect of Monthly Vitamin D Supplementation on Preventing Exacerbations of Asthma or Chronic Obstructive Pulmonary Disease in Older Adults: Post Hoc Analysis of a Randomized Controlled Trial. Nutrients 2021, 13, 521. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020521
Camargo CA Jr., Toop L, Sluyter J, Lawes CMM, Waayer D, Khaw K-T, Martineau AR, Scragg R. Effect of Monthly Vitamin D Supplementation on Preventing Exacerbations of Asthma or Chronic Obstructive Pulmonary Disease in Older Adults: Post Hoc Analysis of a Randomized Controlled Trial. Nutrients. 2021; 13(2):521. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020521
Chicago/Turabian StyleCamargo, Carlos A., Jr., Les Toop, John Sluyter, Carlene M. M. Lawes, Debbie Waayer, Kay-Tee Khaw, Adrian R. Martineau, and Robert Scragg. 2021. "Effect of Monthly Vitamin D Supplementation on Preventing Exacerbations of Asthma or Chronic Obstructive Pulmonary Disease in Older Adults: Post Hoc Analysis of a Randomized Controlled Trial" Nutrients 13, no. 2: 521. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020521