High-Dose Vitamin C in Advanced-Stage Cancer Patients
Abstract
:1. Introduction
2. Over 50 Years of History of the Anti-Cancer Properties of Vitamin C
3. Vitamin C Intravenous Versus Oral Route
4. Biological Properties of Vitamin C
5. Vitamin C Plasma Concentrations in End-Stage Cancer Patients
6. Potential Anticancer Properties of Vitamin C
7. Does High-Dose IVC in Monotherapy Is an Effective Anti-Cancer Agent in Patients with Advanced-Stage Malignancies?
8. Does High-Dose IVC Increase the Effectiveness of Chemotherapy in Patients with Advanced-Stage Cancer?
9. Does High-Dose IVC Reduce the Chemotherapy-Induced Toxicity in Patients with Advanced-Stage Cancer?
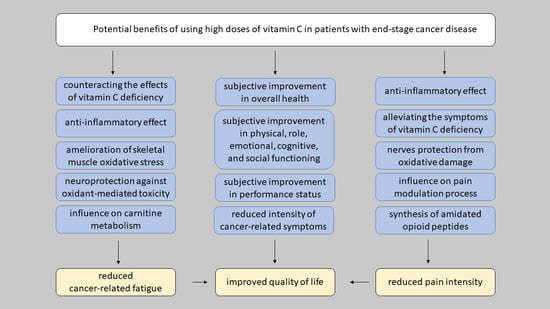
10. Does High-Dose IVC Affect the Quality of Life in Advanced-Stage Cancer Patients?
11. Does High-Dose IVC Affect the Cancer-Related Fatigue in Advanced-Stage Cancer Patients?
12. Potential Analgesic Properties of High-Dose Vitamin C in Cancer-Related Pain
13. Safety of High-Dose Vitamin C Treatment in Advanced-Stage Cancer Patients
14. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, A.Y.; Chen, Q.; Espey, M.G.; Drisko, J.; Levine, M. Vitamin C: Intravenous Use by Complementary and Alternative Medicine Practitioners and Adverse Effects. PLoS ONE 2010, 5, e11414. [Google Scholar] [CrossRef] [Green Version]
- McCormick, W.J. Cancer: The preconditioning factor in pathogenesis; a new etiologic approach. Arch. Pediatr. 1954, 71, 313–322. [Google Scholar]
- McCormick, W.J. Cancer: A collagen disease, secondary to a nutritional deficiency. Arch. Pediatr. 1959, 76, 166–171. [Google Scholar] [PubMed]
- Cameron, E.; Pauling, L. Ascorbic Acid and the Glycosaminoglycans. Oncology 1973, 27, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Pauling, L. The orthomolecular treatment of cancer I. The role of ascorbic acid in host resistance. Chem. Biol. Interact. 1974, 9, 273–283. [Google Scholar] [CrossRef]
- Cameron, E.; Campbell, A. The orthomolecular treatment of cancer II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem. Biol. Interact. 1974, 9, 285–315. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef] [Green Version]
- Murata, A.; Morishige, F.; Yamaguchi, H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int. J. Vitam. Nutr. Res. Suppl. 1982, 23, 103–113. [Google Scholar]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of High-Dose Vitamin C (Ascorbic Acid) Therapy to Benefit Patients with Advanced Cancer. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-Dose Vitamin C versus Placebo in the Treatment of Patients with Advanced Cancer Who Have Had No Prior Chemotherapy. N. Engl. J. Med. 1985, 312, 137–141. [Google Scholar] [CrossRef]
- Graumlich, J.F.; Ludden, T.M.; Conry-Cantilena, C.; Cantilena, L.R.; Wang, Y.; Levine, M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm. Res. 1997, 14, 1133–1139. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Reevaluation of Ascorbate in Cancer Treatment: Emerging Evidence, Open Minds and Serendipity. J. Am. Coll. Nutr. 2000, 19, 423–425. [Google Scholar] [CrossRef]
- Leung, P.Y.; Miyashita, K.; Young, M.; Tsao, C.S. Cytotoxic effect of ascorbate and its derivatives on cultured malignant and nonmalignant cell lines. Anticancer Res. 1993, 13, 475–480. [Google Scholar]
- Bram, S.; Froussard, P.; Guinchard, M.; Jasmin, C.; Augery, Y.; Sinoussi-Barre, F.; Wray, W. Vitamin C preferential toxicity for malignant melanoma cells. Chem. Informationsd. 1980, 11, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Sakagami, H.; Takeda, M. Induction of cell death by ascorbic acid derivatives in human renal carcinoma and glioblastoma cell lines. Anticancer Res. 1999, 19, 3125–3132. [Google Scholar] [PubMed]
- Maramag, C.; Menon, M.; Balaji, K.C.; Reddy, P.G.; Laxmanan, S. Effect of vitamin C on prostate cancer cells in vitro: Effect on cell number, viability, and DNA synthesis. Prostate 1997, 32, 188–195. [Google Scholar] [CrossRef]
- Sakagami, H.; Satoh, K.; Ohata, H.; Takahashi, H.; Yoshida, H.; Iida, M.; Kuribayashi, N.; Sakagami, T.; Momose, K.; Takeda, M. Relationship between ascorbyl radical intensity and apoptosis-inducing activity. Anticancer Res. 1996, 16, 2635–2644. [Google Scholar] [PubMed]
- Padayatty, S.J. Intravenously administered vitamin C as cancer therapy: Three cases. Can. Med. Assoc. J. 2006, 174, 937–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, H.; Flower, G.; Weeks, L.; Cooley, K.; Callachan, M.; McGowan, J.; Skidmore, B.; Kirchner, L.; Seely, D. Intravenous Vitamin C and Cancer. Integr. Cancer Ther. 2014, 13, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Campbell, A.; Jack, T. The orthomolecular treatment of cancer. III. Reticulum cell sarcoma: Double complete regression induced by high-dose ascorbic acid therapy. Chem. Biol. Interactico-Biol. Interact. 1975, 11, 387–393. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538–4542. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A Concentration-Function Approach Yields Pharmacology and Therapeutic Discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Cook, J. Intravenous Vitamin C for Cancer Therapy—Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Robitaille, L.; Zakarian, R.; Melnychuk, D.; Kavan, P.; Agulnik, J.; Cohen, V.; Small, D.; Miller, W.H. High-Dose Intravenous Vitamin C Combined with Cytotoxic Chemotherapy in Patients with Advanced Cancer: A Phase I-II Clinical Trial. PLoS ONE 2015, 10, e0120228. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Højgaard, M.; Andersen, J.T.; Jørgensen, N.R.; Zerahn, B.; Kristensen, B.; Henriksen, T. Weekly ascorbic acid infusion in castration-resistant prostate cancer patients: A single-arm phase II trial. Transl. Androl. Urol. 2017, 6, 517–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef]
- Welsh, J.L.; Wagner, B.A.; van’t Erve, T.J.; Zehr, P.S.; Berg, D.J.; Halfdanarson, T.R.; Yee, N.S.; Bodeker, K.L.; Du, J.; Roberts, L.J.; et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-Dose Parenteral Ascorbate Enhanced Chemosensitivity of Ovarian Cancer and Reduced Toxicity of Chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef]
- Food and Nutrition Board, Institute of Medicine (US): Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Meščić, M.; Gazivoda, K.; Therapeutic, R.-M. Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissers, M.C.M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- May, J.M.; Qu, Z.; Neel, D.R.; Li, X. Recycling of vitamin C from its oxidized forms by human endothelial cells. Biochim. Biophys. Acta Mol. Cell Res. 2003, 1640, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Heitzer, T.; Just, H.; Mu¨nzel, T. Antioxidant Vitamin C Improves Endothelial Dysfunction in Chronic Smokers. Circulation 1996, 94, 6–9. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef]
- Johnston, C.S.; Cox, S.K. Plasma-Saturating Intakes of Vitamin C Confer Maximal Antioxidant Protection to Plasma. J. Am. Coll. Nutr. 2001, 20, 623–627. [Google Scholar] [CrossRef]
- Johnston, C.S.; Dancho, C.L.; Strong, G.M. Orange Juice Ingestion and Supplemental Vitamin C Are Equally Effective at Reducing Plasma Lipid Peroxidation in Healthy Adult Women. J. Am. Coll. Nutr. 2003, 22, 519–523. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Cano, M.P.; de Ancos, B.; Plaza, L.; Olmedilla, B.; Granado, F.; Elez-Martínez, P.; Martín-Belloso, O.; Martín, A. Pulsed electric fields–processed orange juice consumption increases plasma vitamin C and decreases F2-isoprostanes in healthy humans. J. Nutr. Biochem. 2004, 15, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Cano, M.P.; De Ancos, B.; Plaza, L.; Olmedilla, B.; Granado, F.; Martín, A. High-pressurized orange juice consumption affects plasma vitamin C, Antioxidative status and inflammatory markers in healthy humans. J. Nutr. 2003. [Google Scholar] [CrossRef]
- Kuiper, H.C.; Bruno, R.S.; Traber, M.G.; Stevens, J.F. Vitamin C supplementation lowers urinary levels of 4-hydroperoxy-2-nonenal metabolites in humans. Free Radic. Biol. Med. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayment, S.J.; Shaw, J.; Woollard, K.J.; Lunec, J.; Griffiths, H.R. Vitamin C supplementation in normal subjects reduces constitutive ICAM-1 expression. Biochem. Biophys. Res. Commun. 2003, 308, 339–345. [Google Scholar] [CrossRef]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer Disease. Arch. Neurol. 2012, 69. [Google Scholar] [CrossRef] [Green Version]
- Møller, P.; Viscovich, M.; Lykkesfeldt, J.; Loft, S.; Jensen, A.; Poulsen, H.E. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. Eur. J. Nutr. 2004, 43, 267–274. [Google Scholar] [CrossRef]
- Savran, M.; Cicek, E.; Doguc, D.; Asci, H.; Yesilot, S.; Candan, I.; Dagdeviren, B.; Cankara, F.; Oncu, M.; Uğuz, A.; et al. Vitamin C attenuates methotrexate-induced oxidative stress in kidney and liver of rats. Physiol. Int. 2017, 104, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Peng, D.; Ge, G.; Gong, Y.; Zhan, Y.; He, S.; Guan, B.; Li, Y.; Xu, Z.; Hao, H.; He, Z.; et al. Vitamin C increases 5-hydroxymethylcytosine level and inhibits the growth of bladder cancer. Clin. Epigenetics 2018, 10, 94. [Google Scholar] [CrossRef]
- Young, J.I.; Züchner, S.; Wang, G. Regulation of the Epigenome by Vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Klimant, E.; Wright, H.; Rubin, D.; Seely, D.; Markman, M. Intravenous vitamin C in the supportive care of cancer patients: A review and rational approach. Curr. Oncol. 2018, 25, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J. Transl. Med. 2012, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Mikirova, N.; Riordan, N.; Casciari, J. Modulation of cytokines in cancer patients by intravenous ascorbate therapy. Med. Sci. Monit. 2016, 22, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global vitamin c status and prevalence of deficiency: A cause for concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin C deficiency in cancer patients. Palliat. Med. 2005, 19, 17–20. [Google Scholar] [CrossRef]
- Shenoy, N.; Bhagat, T.; Nieves, E.; Stenson, M.; Lawson, J.; Choudhary, G.S.; Habermann, T.; Nowakowski, G.; Singh, R.; Wu, X.; et al. Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J. 2017, 7, e587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riordan, H.D.; Casciari, J.J.; González, M.J.; Riordan, N.H.; Miranda-Massari, J.R.; Taylor, P.; Jackson, J.A. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P. R. Health Sci. J. 2005, 24, 269–276. [Google Scholar] [PubMed]
- Gillberg, L.; Ørskov, A.D.; Nasif, A.; Ohtani, H.; Madaj, Z.; Hansen, J.W.; Rapin, N.; Mogensen, J.B.; Liu, M.; Dufva, I.H.; et al. Oral vitamin C supplementation to patients with myeloid cancer on azacitidine treatment: Normalization of plasma vitamin C induces epigenetic changes. Clin. Epigenetics 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, R.; Faramarzi, E.; Seyedrezazadeh, E.; Mohammad-zadeh, M.; Pourmoghaddam, M. Evaluation of Oxidative Stress, Antioxidant Status and Serum Vitamin C Levels in Cancer Patients. Biol. Trace Elem. Res. 2009, 130, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Torun, M.; Yardim, S.; Gönenç, A.; Sargin, H.; Menevse, A.; Simsek, B. Serum β–carotene, vitamin E, vitamin C and malondialdehyde levels in several types of cancer. J. Clin. Pharm. Ther. 1995, 20, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tripathi, M.; Satyam, A.; Kumar, L. Study of antioxidant levels in patients with multiple myeloma. Leuk. Lymphoma 2009, 50, 809–815. [Google Scholar] [CrossRef]
- Mehdi, W.A.; Zainulabdeen, J.A.; Mehde, A.A. Investigation of the Antioxidant Status in Multiple Myeloma Patients: Effects of Therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3663–3667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emri, S.; Kilickap, S.; Kadilar, C.; Halil, M.G.; Akay, H.; Besler, T. Serum Levels of Alpha-Tocopherol, Vitamin C, Beta-Carotene, and Retinol in Malignant Pleural Mesothelioma. Asian Pac. J. Cancer Prev. 2012, 13, 3025–3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huijskens, M.J.A.J.; Wodzig, W.K.W.H.; Walczak, M.; Germeraad, W.T.V.; Bos, G.M.J. Ascorbic acid serum levels are reduced in patients with hematological malignancies. Results Immunol. 2016, 6, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Parrow, N.L.; Leshin, J.A.; Levine, M. Parenteral Ascorbate As a Cancer Therapeutic: A Reassessment Based on Pharmacokinetics. Antioxid. Redox Signal. 2013, 19, 2141–2156. [Google Scholar] [CrossRef]
- Marcus, S.L.; Petrylak, D.P.; Dutcher, J.P.; Paietta, E.; Ciobanu, N.; Strauman, J.; Wiernik, P.H.; Hutner, S.H.; Frank, O.; Baker, H. Hypovitaminosis C in patients treated with high-dose interleukin 2 and lymphokine-activated killer cells. Am. J. Clin. Nutr. 1991, 54, 1292S–1297S. [Google Scholar] [CrossRef]
- Weijl, N.I.; Hopman, G.D.; Wipkink-Bakker, A.; Lentjes, E.G.W.M.; Berger, H.M.; Cleton, F.J.; Osanto, S. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann. Oncol. 1998, 9, 1331–1337. [Google Scholar] [CrossRef]
- Jonas, C.R.; Puckett, A.B.; Jones, D.P.; Griffith, D.P.; Szeszycki, E.E.; Bergman, G.F.; Furr, C.E.; Tyre, C.; Carlson, J.L.; Galloway, J.R.; et al. Plasma antioxidant status after high-dose chemotherapy: A randomized trial of parenteral nutrition in bone marrow transplantation patients. Am. J. Clin. Nutr. 2000, 72, 181–189. [Google Scholar] [CrossRef]
- Carr, A.C.; Spencer, E.; Das, A.; Meijer, N.; Lauren, C.; MacPherson, S.; Chambers, S.T. Patients Undergoing Myeloablative Chemotherapy and Hematopoietic Stem Cell Transplantation Exhibit Depleted Vitamin C Status in Association with Febrile Neutropenia. Nutrients 2020, 12, 1879. [Google Scholar] [CrossRef]
- Hunnisett, A.; Davies, S.; McLaren-Howard, J.; Gravett, P.; Finn, M.; Gueret-Wardle, D. Lipoperoxides as an index of free radical activity in bone marrow transplant recipients. Biol. Trace Elem. Res. 1995, 47, 125–132. [Google Scholar] [CrossRef]
- Nannya, Y.; Shinohara, A.; Ichikawa, M.; Kurokawa, M. Serial Profile of Vitamins and Trace Elements during the Acute Phase of Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Lykkesfeldt, J.; Poulsen, H.E. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br. J. Nutr. 2010, 103, 1251–1259. [Google Scholar] [CrossRef]
- Benade, L.; Howard, T.; Burk, D. Synergistic Killing of Ehrlich Ascites Carcinoma Cells by Ascorbate and 3-Amino-1,2,4,-triazole. Oncology 1969, 23, 33–43. [Google Scholar] [CrossRef]
- Ristow, M. Oxidative metabolism in cancer growth. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 339–345. [Google Scholar] [CrossRef]
- Ambrose, E.J.; James, A.M.; Lowick, J.H.B. Differences between the Electrical Charge carried by Normal and Homologous Tumour Cells. Nature 1956, 177, 576–577. [Google Scholar] [CrossRef]
- Liotti, F.S.; Menghini, A.R.; Guerrieri, P.; Talesa, V.; Bodo, M. Effects of ascorbic and dehydroascorbic acid on the multiplication of tumor ascites cells in vitro. J. Cancer Res. Clin. Oncol. 1984, 108, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casciari, J.J.; Riordan, H.D.; Miranda-Massari, J.R.; Gonzalez, M.J. Effects of high dose ascorbate administration on L-10 tumor growth in guinea pigs. P. R. Health Sci. J. 2005, 24, 145–150. [Google Scholar]
- Yang, G.; Yan, Y.; Ma, Y.; Yang, Y. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol. Carcinog. 2017, 56, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Verrax, J.; Calderon, P.B. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 2009, 47, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.-H.; Lee, G.; Park, J.-H.; Yu, J.; Park, S.; Yi, S.-Y.; Lee, H.; Hong, Y.; Yang, J.; Lee, S. High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J. Transl. Med. 2009, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.-H.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of Ascorbate-Induced Cytotoxicity in Pancreatic Cancer. Clin. Cancer Res. 2010, 16, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollard, H.B.; Levine, M.A.; Eidelman, O.; Pollard, M. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo 2010, 24, 249–255. [Google Scholar]
- Mamede, A.C.; Pires, A.S.; Abrantes, A.M.; Tavares, S.D.; Gonçalves, A.C.; Casalta-Lopes, J.E.; Sarmento-Ribeiro, A.B.; Maia, J.M.; Botelho, M.F. Cytotoxicity of Ascorbic Acid in a Human Colorectal Adenocarcinoma Cell Line (WiDr): In Vitro and In Vivo Studies. Nutr. Cancer 2012, 64, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [Green Version]
- Jamison, J.M.; Gilloteaux, J.; Nassiri, M.R.; Venugopal, M.; Neal, D.R.; Summers, J.L. Cell cycle arrest and autoschizis in a human bladder carcinoma cell line following Vitamin C and Vitamin K3 treatment. Biochem. Pharmacol. 2004, 67, 337–351. [Google Scholar] [CrossRef]
- Rivière, J.; Ravanat, J.-L.; Wagner, J.R. Ascorbate and H2O2 induced oxidative DNA damage in Jurkat cells. Free Radic. Biol. Med. 2006, 40, 2071–2079. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.-H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, H.J.; Raval, R.R.; Harris, A.L.; Ratcliffe, P.J. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003, 63, 1764–1768. [Google Scholar]
- Kawada, H.; Kaneko, M.; Sawanobori, M.; Uno, T.; Matsuzawa, H.; Nakamura, Y.; Matsushita, H.; Ando, K. High Concentrations of L-Ascorbic Acid Specifically Inhibit the Growth of Human Leukemic Cells via Downregulation of HIF-1α Transcription. PLoS ONE 2013, 8, e62717. [Google Scholar] [CrossRef] [Green Version]
- Miles, S.L.; Fischer, A.P.; Joshi, S.J.; Niles, R.M. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer 2015, 15, 867. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, C.; Dachs, G.U.; Currie, M.J.; Vissers, M.C.M. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic. Biol. Med. 2014, 69, 308–317. [Google Scholar] [CrossRef]
- Gustafson, C.B.; Yang, C.; Dickson, K.M.; Shao, H.; Van Booven, D.; Harbour, J.W.; Liu, Z.-J.; Wang, G. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin. Epigenetics 2015, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate Induces Ten-Eleven Translocation (Tet) Methylcytosine Dioxygenase-mediated Generation of 5-Hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef] [Green Version]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Rao, A.; Lorincz, M.C.; et al. Vitamin C induces Tet-dependent DNA demethylation in ESCs to promote a blastocyst-like state. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef]
- Belin, S.; Kaya, F.; Duisit, G.; Giacometti, S.; Ciccolini, J.; Fontés, M. Antiproliferative Effect of Ascorbic Acid Is Associated with the Inhibition of Genes Necessary to Cell Cycle Progression. PLoS ONE 2009, 4, e4409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, E.J.; Vissers, M.C.M.; Wohlrab, C.; Hicks, K.O.; Strother, R.M.; Bozonet, S.M.; Robinson, B.A.; Dachs, G.U. Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic. Biol. Med. 2016, 99, 451–462. [Google Scholar] [CrossRef]
- Wilkes, J.G.; O’Leary, B.R.; Du, J.; Klinger, A.R.; Sibenaller, Z.A.; Doskey, C.M.; Gibson-Corley, K.N.; Alexander, M.S.; Tsai, S.; Buettner, G.R.; et al. Pharmacologic ascorbate (P-AscH−) suppresses hypoxia-inducible Factor-1α (HIF-1α) in pancreatic adenocarcinoma. Clin. Exp. Metastasis 2018, 35, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Mizuno, H.; Yanagisawa, A. High-dose intravenous vitamin C improves quality of life in cancer patients. Pers. Med. Universe 2012, 1, 49–53. [Google Scholar] [CrossRef]
- Kawada, H.; Sawanobori, M.; Tsuma-Kaneko, M.; Wasada, I.; Miyamoto, M.; Murayama, H.; Toyosaki, M.; Onizuka, M.; Tsuboi, K.; Tazume, K.; et al. Phase I Clinical Trial of Intravenous L-ascorbic Acid Following Salvage Chemotherapy for Relapsed B-cell non-Hodgkin’s Lymphoma. Tokai J. Exp. Clin. Med. 2014, 39, 111–115. [Google Scholar]
- Polireddy, K.; Dong, R.; Reed, G.; Yu, J.; Chen, P.; Williamson, S.; Violet, P.-C.; Pessetto, Z.; Godwin, A.K.; Fan, F.; et al. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Sci. Rep. 2017, 7, 17188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar] [PubMed]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Wagner, B.A.; Cramer-Morales, K.L.; Furqan, M.; Sandhu, S.; Carlisle, T.L.; Smith, M.C.; Abu Hejleh, T.; et al. O2·− and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell 2017, 31, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Raymond, Y.C.F.; Glenda, C.S.L.; Meng, L.K. Effects of High Doses of Vitamin C on Cancer Patients in Singapore: Nine Cases. Integr. Cancer Ther. 2016, 15, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Riordan, H.D.; Riordan, N.H.; Jackson, J.A.; Casciari, J.J.; Hunninghake, R.; González, M.J.; Mora, E.M.; Miranda-Massari, J.R.; Rosario, N.; Rivera, A. Intravenous vitamin C as a chemotherapy agent: A report on clinical cases. P. R. Health Sci. J. 2004, 23, 115–118. [Google Scholar]
- Bahlis, N.J.; McCafferty-Grad, J.; Jordan-McMurry, I.; Neil, J.; Reis, I.; Kharfan-Dabaja, M.; Eckman, J.; Goodman, M.; Fernandez, H.F.; Boise, L.H.; et al. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin. Cancer Res. 2002, 8, 3658–3668. [Google Scholar]
- Abou-Jawde, R.M.; Reed, J.; Kelly, M.; Walker, E.; Andresen, S.; Baz, R.; Karam, M.A.; Hussein, M. Efficacy and Safety Results with the Combination Therapy of Arsenic Trioxide, Dexamethasone, and Ascorbic Acid in Multiple Myeloma Patients: A Phase 2 Trial. Med. Oncol. 2006, 23, 263–272. [Google Scholar] [CrossRef]
- Wu, K.L.; Beksac, M.; Van Droogenbroeck, J.; Amadori, S.; Zweegman, S.; Sonneveld, P. Phase II multicenter study of arsenic trioxide, ascorbic acid and dexamethasone in patients with relapsed or refractory multiple myeloma. Haematologica 2006, 91, 1722–1723. [Google Scholar]
- Berenson, J.R.; Matous, J.; Swift, R.A.; Mapes, R.; Morrison, B.; Yeh, H.S. A Phase I/II Study of Arsenic Trioxide/Bortezomib/Ascorbic Acid Combination Therapy for the Treatment of Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2007, 13, 1762–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventafridda, V. According to the 2002 WHO Definition of Palliative Care. Palliat. Med. 2006, 20, 159. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H. Measuring Health-Related Quality of Life. Ann. Intern. Med. 1993, 118, 622–629. [Google Scholar] [CrossRef]
- Bottomley, A. The Cancer Patient and Quality of Life. Oncologist 2002, 7, 120–125. [Google Scholar] [CrossRef]
- Saxena, S.; Orley, J. Quality of life assessment: The World Health Organization perspective. Eur. Psychiatry 1997, 12, 263s–266s. [Google Scholar] [CrossRef]
- Aaronson, N.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.; Filiberti, A.; Flechtner, H.; Fleishman, S.; De Haes, J.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Nayak, M.; George, A.; Vidyasagar, M.; Mathew, S.; Nayak, S.; Nayak, B.; Shashidhara, Y.; Kamath, A. Quality of life among cancer patients. Indian J. Palliat. Care 2017, 23, 445. [Google Scholar] [CrossRef]
- Bovero, A.; Leombruni, P.; Miniotti, M.; Rocca, G.; Torta, R. Spirituality, quality of life, psychological adjustment in terminal cancer patients in hospice. Eur. J. Cancer Care 2016, 25, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.H.; Jung, G.C.; Song, K.J. Changes of Terminal Cancer Patients’ Health-related Quality of Life after High Dose Vitamin C Administration. J. Korean Med. Sci. 2007, 22, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Vissers, M.C.M.; Cook, J. Relief from cancer chemotherapy side effects with pharmacologic vitamin C. N. Z. Med. J. 2014, 127, 66–70. [Google Scholar] [PubMed]
- Carr, A.C.; Vissers, M.C.M.; Cook, J. Parenteral vitamin C for palliative care of terminal cancer patients. N. Z. Med. J. 2014, 127, 84–86. [Google Scholar] [PubMed]
- Günes-Bayir, A.; Kiziltan, H.S. Palliative Vitamin C Application in Patients with Radiotherapy-Resistant Bone Metastases: A Retrospective Study. Nutr. Cancer 2015, 67, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C.I. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Horneber, M.; Fischer, I.; Dimeo, F.; Rüffer, J.; Weis, J. Cancer-related fatigue: Epidemiology, pathogenesis, diagnosis, and treatment. Dtsch. Arztebl. Int. 2012, 109, 161–171. [Google Scholar] [PubMed]
- Lawrence, D.P. Evidence Report on the Occurrence, Assessment, and Treatment of Fatigue in Cancer Patients. J. Natl. Cancer Inst. Monogr. 2004, 32, 40–50. [Google Scholar] [CrossRef]
- Butt, Z.; Rosenbloom, S.K.; Abernethy, A.P.; Beaumont, J.L.; Paul, D.; Hampton, D.; Jacobsen, P.B.; Syrjala, K.L.; Von Roenn, J.H.; Cella, D. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. JNCCN J. Natl. Compr. Cancer Netw. 2008, 6, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Suh, S.-Y.; Bae, W.K.; Ahn, H.-Y.; Choi, S.-E.; Jung, G.-C.; Yeom, C.H. Intravenous Vitamin C administration reduces fatigue in office workers: A double-blind randomized controlled trial. Nutr. J. 2012, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Gholami, M.; Najafizadeh, H.; Teimouri, H.; Ardalan, A.; Pooria, A.; Tarrahi, M.J. The combined effect of vitamin C and omega-3 polyunsaturated fatty acids on fatigue following coronary artery bypass graft surgery: A triple-blind clinical trial. J. Complement. Integr. Med. 2019, 16. [Google Scholar] [CrossRef]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, L.A.A.; St. Clair, D.K. Chemotherapy-Induced Weakness and Fatigue in Skeletal Muscle: The Role of Oxidative Stress. Antioxid. Redox Signal. 2011, 15, 2543–2563. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Vissers, M.C.M.; Cook, J.S. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, J.M. Vitamin C Transport and Its Role in the Central Nervous System. In Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2012; Volume 56, pp. 85–103. ISBN 9789400721999. [Google Scholar]
- van den Beuken-van Everdingen, M.H.J.; Hochstenbach, L.M.J.; Joosten, E.A.J.; Tjan-Heijnen, V.C.G.; Janssen, D.J.A. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Dionne, C.E.; Laurin, D.; Desrosiers, T.; Abdous, B.; Le Sage, N.; Frenette, J.; Mondor, M.; Pelletier, S. Serum vitamin C and spinal pain. Pain 2016, 157, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Kiziltan, H.S.; Bayir, A.G.; Demirtas, M.; Meral, I.; Taspinar, O.; Eris, A.H.; Aydin, T.; Mayadagli, A. Ascorbic-acid Treatment for Progressive Bone Metastases After Radiotherapy: A Pilot Study. Altern. Ther. Health Med. 2014, 20, 16–20. [Google Scholar]
- Park, J.-M.; Kim, C.K.; Lee, H.C.; Jung, H.; Choi, K.-U.; Hong, S.W.; Lim, D.G.; Baek, W.-Y.; Kwak, K.-H. Antiallodynic effects of vitamin C and vitamin E in chronic post-ischemia pain rat model. Korean J. Anesthesiol. 2013, 65, 442. [Google Scholar] [CrossRef] [Green Version]
- Kallner, A. Influence of vitamin C status on the urinary excretion of catecholamines in stress. Hum. Nutr. Clin. Nutr. 1983, 37, 405–411. [Google Scholar] [PubMed]
- Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-Y.; Chang, C.-Y.; Feng, P.-H.; Chu, C.-C.; So, E.C.; Hu, M.-L. Plasma Vitamin C Is Lower in Postherpetic Neuralgia Patients and Administration of Vitamin C Reduces Spontaneous Pain but Not Brush-evoked Pain. Clin. J. Pain 2009, 25, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, D.J.; Na, C.H.; Shin, B.S. A Study of Intravenous Administration of Vitamin C in the Treatment of Acute Herpetic Pain and Postherpetic Neuralgia. Ann. Dermatol. 2016, 28, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-K.; Lin, Y.-T.; Hung, K.-C.; Chang, C.-Y.; Wu, Z.-F.; Hu, M.-L.; Chen, J.-Y. Plasma Vitamin C Concentrations Were Negatively Associated with Tingling, Prickling or Pins and Needles Sensation in Patients with Postherpetic Neuralgia. Nutrients 2020, 12, 2384. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shen, L.; Yu, X.; Ma, C.; Huang, Y. Vitamin C enhances the analgesic effect of gabapentin on rats with neuropathic pain. Life Sci. 2016, 157, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Riffel, A.P.K.; de Souza, J.A.; Maria do Carmo, Q.S.; Horst, A.; Scheid, T.; Kolberg, C.; Belló-Klein, A.; Partata, W.A. Systemic administration of vitamins C and E attenuates nociception induced by chronic constriction injury of the sciatic nerve in rats. Brain Res. Bull. 2016, 121, 169–177. [Google Scholar] [CrossRef]
- Lu, R.; Kallenborn-Gerhardt, W.; Geisslinger, G.; Schmidtko, A. Additive Antinociceptive Effects of a Combination of Vitamin C and Vitamin E after Peripheral Nerve Injury. PLoS ONE 2011, 6, e29240. [Google Scholar] [CrossRef]
- Saffarpour, S.; Nasirinezhad, F. Functional interaction between N-methyl-D-aspartate receptor and ascorbic acid during neuropathic pain induced by chronic constriction injury of the sciatic nerve. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 601–608. [Google Scholar] [CrossRef]
- Pinkerton, E.; Good, P.; Gibbons, K.; Hardy, J. An open-label pilot study of oral vitamin C as an opioid-sparing agent in patients with chronic pain secondary to cancer. Support. Care Cancer 2017, 25, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Steinberg, M.; Bower, J. Ascorbic Acid-Induced Hemolysis in G-6-PD Deficiency. Ann. Intern. Med. 1975, 82, 810. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Kelsey, H.; Richards, J.D.M. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ 1993, 306, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, N.; Kawaguchi, T.; Horikawa, K.; Nagakura, S.; Hidaka, M.; Kagimoto, T.; Takatsuki, K.; Nakakuma, H. Haemolysis induced by ascorbic acid in paroxysmal nocturnal haemoglobinuria. Lancet 1994, 343, 357. [Google Scholar] [CrossRef]
- Lawton, J.M. Acute Oxalate Nephropathy After Massive Ascorbic Acid Administration. Arch. Intern. Med. 1985, 145, 950. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Thomson, C.; Bailey, R.R.; McDiarmid, S.; Gardner, J. Acute oxalate nephropathy after a massive intravenous dose of vitamin C. Aust. N. Z. J. Med. 1994, 24, 410–411. [Google Scholar] [CrossRef] [PubMed]
| Type of Study | Patients | Intervention | Outcome | References |
|---|---|---|---|---|
| Prospective | 39 terminal cancer patients | 10 g IVC twice with a 3-day interval for one week, followed by oral intake of 4 g daily for one week | Improved QoL assessed by the EORTC QLQ-C30 questionnaire:
| Yeom et al. [120] |
| Prospective | 24 patients with advanced cancer and hematological malignancy refractory to standard therapy | IVC three times a week at fixed doses 0.4, 0.6, 0.9 and 1.5 g/kg for average 10 weeks | QoL assessed by the FACT-G questionnaire:
| Hoffer et al. [29] |
| Prospective | 60 patients with cancer, anti-cancer therapy administered in 34 patients | 4 weeks of IVC therapy, median single dose 50 g (range 25–100 g) | Improved QoL assessed by the EORTC QLQ-C30 questionnaire:
| Takahashi et al. [102] |
| Prospective | 17 patients with advanced solid tumors refractory to standard therapy | IVC for 4 consecutive days a week for 4 weeks, starting at 30 g/m2, a dose was increased by 20 g/m2 until a maximum tolerated dose (110 g/m2) | Improved QoL assessed by the EORTC QLQ-C30 questionnaire:
| Stephenson et al. [28] |
| Prospective | 23 patients with metastatic castration-resistant prostate cancer | IVC once weekly: 5 g in 1st week, 30 g in 2nd week, 60 g in 3–12 weeks | QoL assessed by the EORTC QLQ-C30 questionnaire:
| Nielsen et al. [27] |
| Controlled retrospective | 125 patients with breast cancer on anti-tumor therapy (study group n = 53 ; control group n = 72) | Study group: IVC 7.5 g once a week during adjuvant therapies for a minimum 4 weeks Controls: no IVC during adjuvant therapies | Improved performance status assessed by the Karnofsky index and the ECOG scale during the 6 months of study * and the next 6 months of aftercare * Reduced intensity of complains (study group vs. controls):
| Vollbracht et al. [105] |
| Controlled retrospective | 39 patients with bone metastases, radiotherapy-resistant (n = 15 on chemotherapy; n = 15 on IVC therapy; n = 9 controls) | IVC group: 2.5 g IVC during pain | Improved performance status assessed by the ECOG scale in 27% of IVC group and 7% of the chemotherapy group, while worsened in the control group | Günes-Bayir et al. [123] |
| Case study | A 45-year old female with recurrent breast cancer | IVC 50 g twice a week for 4 weeks | Improved QoL assessed by the EORTC QLQ-C30 questionnaire:
| Carr et al. [121] |
| Case study | A 81-year-old male with recurrent pulmonary angiosarcoma | IVC 30 g daily for 1 week | Improved QoL assessed by the EORTC QLQ-C30 questionnaire:
| Carr et al. [122] |
| Study Type | Characteristics of Study Participants | Intervention | Outcome | References |
|---|---|---|---|---|
| Prospective | 39 terminal cancer patients | 10 g IVC twice with a 3-day interval in the first week, followed by oral intake of 4 g daily for one week | Reduced intensity of pain after IVC treatment | Yeom et al. [120] |
| Prospective | 60 patients with newly diagnosed cancer, anti-cancer therapy administered in 34 patients | 4 weeks of IVC therapy, median single dose 50 g (range 25–100 g) | Reduced intensity of pain after IVC treatment | Takahashi et al. [102] |
| Prospective | 17 patients with advanced solid tumors refractory to standard therapy | IVC for 4 consecutive days a week for 4 weeks, starting at 30 g/m2, increased until a maximum tolerated dose (110 g/m2) | Reduced intensity of pain during IVC with complete pain relief after 4 weeks of IVC therapy | Stephenson et al. [28] |
| Controlled retrospective | 39 patients with bone metastases, radiotherapy-resistant (n = 15 on chemotherapy; n = 15 on IVC therapy; n = 9 controls) | IVC group: 2.5 g IVC during pain | Median 50% reduction in pain intensity in IVC group | Günes-Bayir et al. [123] |
| Uncontrolled retrospective | 11 cancer patients with bone metastases, unresponsive to standard cancer treatment | IVC 2.5 g once weekly for 3–10 weeks | Mean 55% reduction in pain intensity | Kiziltan et al. [138] |
| Case study | A female aged 53 with breast carcinoma with visceral and skeletal metastases, after mastectomy, radiotherapy, and hormonotherapy, terminal state with severe pain requiring opioids | 5 g IVC for 7 days, followed by 8 g daily for 70 days orally (total 595 g) | Complete relief from bone pain Reduced need for opioids | Cameron and Campbell [7] |
| A male aged 44 with poorly differentiated transitional cell cancer of bladder with bone metastases, intense pain inadequately controlled by opioids | 10 g IVC for 10 days, followed by 10 g daily for 24 days orally (total 340 g) | Complete relief from bone pain No further need for opioids | Cameron and Campbell [7] | |
| A female 49 with disseminated carcinoma of unknown origin, innumerable osteolytic bone metastases, severe bone pain | 10 g IVC for 7 days, followed by 10 g daily for 27 days orally (total 340 g) | Complete relief from bone pain | Cameron and Campbell [7] | |
| A male aged 49 with large malignant tumor of right temporal lobe, unknown histology, intolerable headache | 10 g IVC for 11 days, followed by 10 g daily for 2 days orally (total 360 g) | Significant relief from headache | Cameron and Campbell [7] | |
| Case study | A female aged 45 with recurrent breast cancer | 50 g IVC twice a week for 4 weeks | Reduction in pain intensity | Carr et al. [121] |
| Case study | A male aged 81 with recurrent pulmonary angiosarcoma | 30 g IVC daily for 1 week | Reduction in pain intensity | Carr et al. [122] |
| Patients | Intervention | Adverse Effects | References |
|---|---|---|---|
| 24 terminal cancer patients | 150–710 mg/kg/day IVC for up to 8 weeks | Frequently reported: nausea, edema, dry mouth or skin Grade 3 adverse events: kidney stone (n = 1), hypokalemia (n = 1) | Riordan et al. [58] |
| 24 patients with advanced metastatic solid tumor or hematological malignancy refractory to standard therapy | IVC 3 times a week at fixed doses 0.4, 0.6, 0.9 and 1.5 g/kg for average 10 weeks | Mild subjective symptoms: nausea (n = 3), diarrhea (n = 2), headache (n = 2), dizziness (n = 2), fatigue (n = 2), facial flushing (n = 2), abdominal cramps (n = 1), vomiting (n = 1), perspiration (n = 1) | Hoffer et al. [29] |
| 14 patients with metastatic stage IV pancreatic cancer | IVC 50–100 g/d three times a week for 8 weeks along with standard treatment of gemcitabine and erlotinib | Frequently reported: dizziness, nausea Grade 1–2 adverse events: low platelet count (n = 8), low hemoglobin count (n = 1), low neutrophil count (n = 1), hyperglycemia (n = 1), gastrointestinal discomfort (n = 1), conjunctival infection (n = 1), ascites (n = 1) Grade 3–4 adverse events: internal bleeding (n = 1), pulmonary embolism (n = 2), hospitalization due to anemia and UTI (n = 1), ileus (n = 1) Grade 5 adverse events: death from disease progression (n = 1) | Monti et al. [32] |
| 11 patients with advanced pancreatic cancer | IVC 15–125 g/d twice a week for 8 weeks along with standard treatment (gemcitabine) | Frequently reported grade 1–2 adverse events: nausea (n = 6), diarrhea (n = 4), dry mouth (n = 4) Grade 3–4 adverse effects: elevated plasma GGT (n = 2), hypokalemia (n = 1), leukopenia (n = 1), lymphopenia (n = 1), neutropenia (n = 2), thrombocytopenia (n = 1) | Welsh et al. [30] |
| 17 patients with advanced solid tumors refractory to standard therapy | IVC for 4 consecutive days a week for 4 weeks, starting at 30 g/m2, doses increased by 20 g/m2 until a maximum tolerated dose (110 g/m2) | Grade 1–2 adverse events: HT (n = 4), hypernatremia (n = 2), hypoalbuminemia (n = 1), hypokalemia (n = 1), hyperglycemia (n = 1), hypercalcemia (n = 1), increased creatinine (n = 1), elevated plasma LDH (n = 1), proteinuria (n = 1), bacteremia (n = 1), granular casts (n = 1), lower back pain (n = 1), tumor fever (n = 1), pedal edema (n = 1), headache (n = 1), peripheral neuropathy (n = 1) Grade 3–4 adverse events: hypokalemia (n = 2), hypernatremia (n = 2), anemia (n = 2), headache (n = 1) | Stephenson et al. [28] |
| 14 patients with advanced cancer | IVC 1.5 g/kg body weight 2 or 3 times a week in combination with chemotherapy | Frequently reported mild adverse events: thirst, increased urinary flow Others: nausea and occasional vomiting (n = 1), thirst and unpleasant sensation in the upper abdomen (n = 1), chills, thirst, headache, leg edema (n = 1) | Hoffer et al. [26] |
| 23 patients with metastatic castration-resistant prostate cancer | IVC once weekly: 5 g in 1st week, 30 g in 2nd week, 60 g in 3–12 weeks | Grade 1–2 adverse events: anemia (n = 7), HT (n = 5), UTI (n = 4), elevation of plasma aminotransferase (n = 3), ↓GFR (n = 3), flu-like symptoms (n = 3), limb pain (n = 3), musculoskeletal lesion (n = 2), shortness of breath (n = 2), pneumonia (n = 1), diarrhea (n = 1), dry eyes (n = 1), osteoporotic fracture (n = 1), pre-syncope (n = 1), leukemia (n = 1), AF (n = 1), elevated plasma bilirubin (n = 1), hydronephrosis (n = 1), hypercalcemia (n = 1), hyponatremia (n = 1) Grade 3–4 adverse event: pulmonary embolism (n = 1), pneumonia (n = 1) | Nielsen et al. [27] |
| Myths | Facts |
|---|---|
| High-dose IVC is a potent anticancer treatment because of its proven effectiveness in preclinical in vitro and animal studies | No consistent evidence for anti-cancer efficacy of high dose IVC therapy in patients with advanced-stage cancer in terms of objective tumor-related response or improved survival outcomes. |
| High-dose IVC treatment enhances effectiveness of conventional anticancer therapy | No reliable evidence for increased effectiveness of combined IVC/conventional therapy compared to standard chemotherapy. |
| High-dose IVC treatment reduces chemotherapy-induced toxicity | No reliable evidence for decreased chemotherapy-induced toxicity after combined IVC/conventional therapy compared to standard chemotherapy. |
| High-dose IVC reduces fatigue in cancer patients | Positive effect of high-dose IVC on cancer-related fatigue comprises various factors and it can be more expressed in patients with basically better performance status and in those with chemotherapy-related fatigue. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasowska-Nowak, A.; Nowak, P.J.; Ciałkowska-Rysz, A. High-Dose Vitamin C in Advanced-Stage Cancer Patients. Nutrients 2021, 13, 735. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030735
Zasowska-Nowak A, Nowak PJ, Ciałkowska-Rysz A. High-Dose Vitamin C in Advanced-Stage Cancer Patients. Nutrients. 2021; 13(3):735. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030735
Chicago/Turabian StyleZasowska-Nowak, Anna, Piotr Jan Nowak, and Aleksandra Ciałkowska-Rysz. 2021. "High-Dose Vitamin C in Advanced-Stage Cancer Patients" Nutrients 13, no. 3: 735. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030735