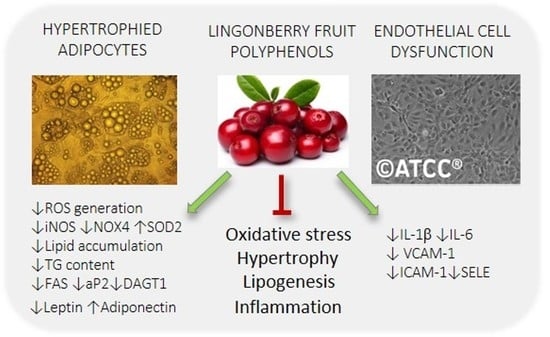
ROS Modulating Effects of Lingonberry (Vaccinium vitis-idaea L.) Polyphenols on Obese Adipocyte Hypertrophy and Vascular Endothelial Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Anthocyanin and Non-Anthocyanin Polyphenol Fractions
2.2. Polyphenol Identification and Quantification in ACN and PP Fractions
2.3. 3T3-L1 Adipocyte Culture and Treatment
2.4. HUVEC Culture and Treatment
2.5. Cell Viability Assay
2.6. Determination of Intracellular ROS Production
2.7. Measurement of Intracellular Lipid Content
2.8. RNA Extraction and Real-Time PCR Analysis
2.9. Determination of Adipokine Production
2.10. Statistical Analysis
3. Results and Discussion
3.1. Polyphenol Composition in the Lingonberry ACN and PP Fractions
3.2. The Effect of PP and ACN Fractions on ROS Generation in Hypertrophied 3T3-L1 Adipocytes
3.3. Effect of ACN and PP Fractions on Lipid Accumulation in Hypertrophied 3T3-L1 Adipocytes
3.4. Effect of ACN and PP Fractions on Adipokine and Inflammatory Cytokine Expression in Hypertrophied 3T3-L1 Adipocytes
3.5. The Effects of PP and ACN Fractions on TNF-α-Induced Endothelial Dysfunction
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barroso, T.A.; Marins, L.B.; Alves, R.; Gonçalves, A.C.S.; Barroso, S.G.; Rocha, G.D.S. Association of central obesity with the incidence of cardiovascular diseases and risk factors. Int. J. Cardiovasc. Sci. 2017, 30, 416–424. [Google Scholar] [CrossRef]
- Lafontan, M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2013, 40, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Al Suwaidi, J.; Higano, S.T.; Holmes, D.R., Jr.; Lennon, R.; Lerman, A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J. Am. Coll. Cardiol. 2001, 37, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.K.; Zhou, Z.; Zhang, J.; Zeng, R.; Wu, J.; Eitzman, D.T.; Chen, Y.E.; Chang, L. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1621–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Munzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001, 104, 2673–2678. [Google Scholar] [CrossRef] [Green Version]
- Caballero, A.E. Endothelial dysfunction in obesity and insulin resistance: A road to diabetes and heart disease. Obes. Res. 2003, 11, 1278–1289. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A. Current evidence on the health-beneficial effects of berry fruits in the prevention and treatment of metabolic syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 446–452. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the triterpenoid content of berries and leaves of lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Zielińska-Wasielica, J.; Olkowicz, M. Inhibitory effects of lingonberry (Vaccinium vitis-idaea L.) fruit extract on obesity-induced inflammation in 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Funct. Foods 2019, 54, 371–380. [Google Scholar] [CrossRef]
- Eid, H.M.; Ouchfoun, M.; Brault, A.; Vallerand, D.; Musallam, L.; Arnason, J.T.; Haddad, P.S. Lingonberry (Vaccinium vitis-idaea L.) exhibits antidiabetic activities in a mouse model of diet-induced obesity. Evid. Based Complement. Alternat. Med. 2014, 2014, 645812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit adipogenesis and lipogenesis in 3T3-L1 cells. Food Chem. 2014, 148, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, J.W.; Cha, Y.N.; Kim, C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J. Immunoass. Immunochem. 2006, 27, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Galili, O.; Versari, D.; Sattler, K.J.; Olson, M.L.; Mannheim, D.; McConnell, J.P.; Chade, A.R.; Lerman, L.O.; Lerman, A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H904–H911. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases. The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Perreault, M.; Marette, A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat. Med. 2001, 7, 1138–1143. [Google Scholar] [CrossRef]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, Y.C.; Chang, W.T.; Hsu, C.L. Effects of polyphenolic compounds on tumor necrosis factor-α (TNF-α)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J. Agric. Food Chem. 2011, 59, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M. Cyanidin 3-glucoside protects 3T3-L1 adipocytes against H2O2- or TNF-alpha-induced insulin resistance by inhibiting c-Jun NH2-terminal kinase activation. Biochem. Pharmacol. 2008, 75, 1393–1401. [Google Scholar] [CrossRef]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; Lefebvre d’Hellencourt, C.; Gonthier, M.P.; Robert-Da Silva, C. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-κB genes. J. Inflamm. 2015, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Mane, C.; Loonis, M.; Juhel, C.; Dufour, C.; Malien-Aubert, C. Food grade lingonberry extract: Polyphenolic composition and in vivo protective effect against oxidative stress. J. Agric. Food Chem. 2011, 59, 3330–3339. [Google Scholar] [CrossRef]
- Yang, X.F.; Qiu, Y.Q.; Wang, L.; Gao, K.G.; Jiang, Z.Y. A high-fat diet increases body fat mass and up-regulates expression of genes related to adipogenesis and inflammation in a genetically lean pig. J. Zhejiang Univ. Sci. B. 2018, 19, 884–894. [Google Scholar] [CrossRef]
- Chen, H.C.; Farese, R.V., Jr. Inhibition of triglyceride synthesis as a treatment strategy for obesity: Lessons from DGAT1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 482–486. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berndt, J.; Kovacs, P.; Ruschke, K.; Klöting, N.; Fasshauer, M.; Schön, M.R.; Körner, A.; Stumvoll, M.; Blüher, M. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia 2007, 50, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.C.; Jensen, R.; Myers, H.M.; Eckel, R.H.; Farese, R.V., Jr. Obesity resistance and enhanced glucose metabolism in mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. J. Clin. Investig. 2003, 111, 1715–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowski, L.; Hotamisligil, G.S. Fatty acid binding proteins—The evolutionary crossroads of inflammatory and metabolic responses. J. Nutr. 2004, 134, 2464S–2468S. [Google Scholar] [CrossRef] [Green Version]
- Naik, R.; Obiang-Obounou, B.W.; Kim, M.; Choi, Y.; Lee, H.S.; Lee, K. Therapeutic strategies for metabolic diseases: Small-molecule diacylglycerol acyltransferase (DGAT) inhibitors. Chem. Med. Chem. 2014, 9, 2410–2424. [Google Scholar] [CrossRef]
- Kondo, H.; Hashizume, K.; Shibuya, Y.; Hase, T.; Murase, T. Identification of diacylglycerol acyltransferase inhibitors from Rosa centifolia petals. Lipids 2011, 46, 691–700. [Google Scholar] [CrossRef]
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef]
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of beneficial metabolic effects of berries in high-fat fed C57BL/6J mice. J. Nutr. Metab. 2014, 403041. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Anderson, R.A. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Cheng, K.K.; Vanhoutte, P.M.; Lam, K.S.; Xu, A. Vascular effects of adiponectin: Molecular mechanisms and potential therapeutic intervention. Clin. Sci. 2008, 114, 361–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.J.; Sun, H.W.; Zhu, F.L.; Chen, L.; Rong, Y.Y.; Zhang, Y.; Zhang, M. Local adiponectin treatment reduces atherosclerotic plaque size in rabbits. J. Endocrinol. 2007, 193, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Lonnqvist, F.; Nordfors, L.; Jansson, M.; Thorne, A.; Schalling, M.; Arner, P. Leptin secretion from adipose tissue in women: Relationship to plasma levels and gene expression. J. Clin. Investig. 1997, 99, 2398–2404. [Google Scholar] [CrossRef] [Green Version]
- Beltowski, J.; Wójcicka, G.; Marciniak, A.; Jamroz, A. Oxidative stress, nitric oxide production, and renal sodium handling in leptin-induced hypertension. Life Sci. 2004, 74, 2987–3000. [Google Scholar] [CrossRef]
- Bouloumie, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999, 13, 1231–1238. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- McDermott, M.M.; Liu, K.; Ferrucci, L.; Tian, L.; Guralnik, J.M.; Tao, H.; Ridker, P.M.; Criqui, M.H. Relation of interleukin-6 and vascular cellular adhesion molecule-1 levels to functional decline in patients with lower extremity peripheral arterial disease. Am. J. Cardiol. 2011, 107, 1392–1398. [Google Scholar] [CrossRef] [Green Version]
- Baum, J.I.; Howard, L.R.; Prior, R.L.; Lee, S.O. Effect of Aronia melanocarpa (Black Chokeberry) supplementation on the development of obesity in mice fed a high-fat diet. J. Berry Res. 2016, 6, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.; Hager, A.; Wilkes, S.; Howard, L. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol. Nutr. Food Res. 2009, 53, 1406–1418. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef]
- Tucakovic, L.; Colson, N.; Santhakumar, A.B.; Kundur, A.R.; Shuttleworth, M.; Singh, I. The effects of anthocyanins on body weight and expression of adipocyte’s hormones: Leptin and adiponectin. J. Funct. Foods 2018, 45, 173–180. [Google Scholar] [CrossRef]
- Vugic, L.; Colson, N.; Nikbakht, E.; Gaiz, A.; Holland, O.J.; Kundur, A.R.; Singh, I. Anthocyanin supplementation inhibits secretion of pro-inflammatory cytokines in overweight and obese individuals. J. Funct. Foods 2020, 64, 103596. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The Influence of Supplementation of Anthocyanins on Obesity-Associated Comorbidities: A Concise Review. Foods 2020, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Quyyumi, A.A. Endothelial function in health and disease: New insights into the genesis of cardiovascular disease. Am. J. Med. 1998, 105, 32S–39S. [Google Scholar] [CrossRef]
- Fernández-Alfonso, M.S.; Somoza, B.; Tsvetkov, D.; Kuczmanski, A.; Dashwood, M.; Gil-Ortega, M. Role of Perivascular Adipose Tissue in Health and Disease. Compr. Physiol. 2017, 8, 23–59. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Ciotola, M.; Sasso, F.C.; Cozzolino, D.; Saccomanno, F.; Assaloni, R.; Ceriello, A.; Giugliano, D. Effect of a single high-fat meal on endothelial function in patients with the metabolic syndrome: Role of tumor necrosis factor-α. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 274–279. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Youdim, K.A.; McDonald, J.; Kalt, W.; Joseph, J.A. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J. Nutr. Biochem. 2002, 13, 282–288. [Google Scholar] [CrossRef]
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Low-calorie cranberry juice supplementation reduces plasma oxidized LDL and cell adhesion molecule concentrations in men. Br. J. Nutr. 2008, 99, 352–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziccardi, P.; Nappo, F.; Giugliano, G.; Esposito, K.; Marfella, R.; Cioffi, M.; D’Andrea, F.; Molinari, A.M.; Giugliano, D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002, 105, 804–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| (A) | |||||
| Compound | RT | Precursor Ion (m/z) | Ionization Mode | Product Ion (m/z) | Contribution |
| (min) | (%) | ||||
| Cyanidin-3-O-galactoside | 16.25 | 449.1126 | (+) | 287.0611 | 82.5 |
| Cyanidin-3-O-glucoside | 17.17 | 449.1124 | (+) | 287.0589 | 4.5 |
| Cyanidin-3-O-arabinoside | 18.24 | 419.1021 | (+) | 287.0607 | 13 |
| (B) | |||||
| Compound | RT | Precursor Ion (m/z) | Ionization Mode | Product Ion (m/z) | Contribution |
| (min) | (%) | ||||
| Flavan-3-ols | 40.4 | ||||
| B-type procyanidin dimer | 8.46 | 577.134 | (−) | 407.0763 | 6.3 |
| (+)/(−)-Catechin | 10.49 | 289.1139 | (−) | 245.1203 | 8.3 |
| (+)/(−)-Epicatechin | 12.42 | 289.114 | (−) | 245.121 | 4.2 |
| B-type procyanidin dimer | 13.16 | 577.1338 | (−) | 407.0762 | 1.6 |
| B-type procyanidin dimer | 13.74 | 577.1349 | (−) | 407.0769 | 3.1 |
| A-type procyanidin dimer | 19.36 | 575.1195 | (−) | 449.0887 | 6.2 |
| A-type procyanidin trimer | 22.31 | 863.1829 | (−) | 575.1217 | 10.7 |
| Hydroxycinnamic acid derivatives | 22.8 | ||||
| 3-O-Caffeoylquinic acid | 12.42 | 353.134 | (−) | 191.0907 | 8.1 |
| Ferulic acid−hexoside | 13.71 | 355.1037 | (−) | 193.05 | 2.9 |
| Ferulic acid−hexoside | 14.14 | 355.1041 | (−) | 193.0503 | 2.6 |
| 2′-O-Caffeoylarbutin | 19.36 | 433.1137 | (−) | 179.0337 | 5 |
| Ferulic acid−hexoside | 21.7 | 355.1031 | (−) | 193.0509 | 2.5 |
| Coumaroyl-hexose−hydroxyphenol | 22.25 | 417.1065 | (−) | 163.0396 | 1.8 |
| Flavonols | 31 | ||||
| Quercetin-3-O-galactoside | 23.46 | 463.0874 | (−) | 301.0334 | 7.8 |
| Quercetin-3-O-glucoside | 24.17 | 463.0882 | (−) | 301.0354 | 1.3 |
| Quercetin-3-O-xyloside | 24.85 | 433.0777 | (−) | 301.0336 | 1.9 |
| Quercetin-3-O-arabinofuranoside | 27.05 | 433.0776 | (−) | 301.0327 | 6.3 |
| Quercetin-3-O-rhamnoside | 27.87 | 447.0935 | (−) | 301.0349 | 8.1 |
| Quercetin | 34.37 | 301.0356 | (−) | 151.0031 | 5.6 |
| Anthocyanins | 5.8 | ||||
| Cyanidin-3-O-galactoside | 16.28 | 449.1126 | (+) | 287.0611 | 2.6 |
| Cyanidin-pentoside | 21.33 | 419.1033 | (+) | 287.0618 | 2 |
| Cyanidin 3-O-(6″-acetyl)-glucoside | 23.82 | 491.1507 | (+) | 287.0621 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, K.; Dembczyński, R.; Gołąbek, A.; Olkowicz, M.; Olejnik, A. ROS Modulating Effects of Lingonberry (Vaccinium vitis-idaea L.) Polyphenols on Obese Adipocyte Hypertrophy and Vascular Endothelial Dysfunction. Nutrients 2021, 13, 885. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030885
Kowalska K, Dembczyński R, Gołąbek A, Olkowicz M, Olejnik A. ROS Modulating Effects of Lingonberry (Vaccinium vitis-idaea L.) Polyphenols on Obese Adipocyte Hypertrophy and Vascular Endothelial Dysfunction. Nutrients. 2021; 13(3):885. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030885
Chicago/Turabian StyleKowalska, Katarzyna, Radosław Dembczyński, Agata Gołąbek, Mariola Olkowicz, and Anna Olejnik. 2021. "ROS Modulating Effects of Lingonberry (Vaccinium vitis-idaea L.) Polyphenols on Obese Adipocyte Hypertrophy and Vascular Endothelial Dysfunction" Nutrients 13, no. 3: 885. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030885