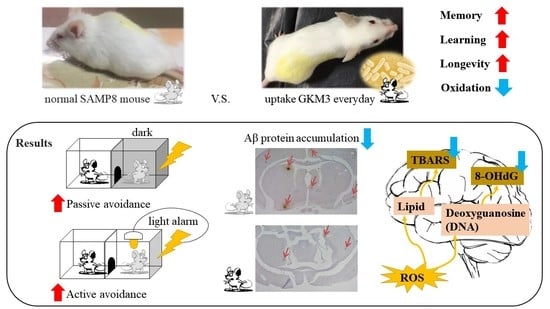
Lactobacillus plantarum GKM3 Promotes Longevity, Memory Retention, and Reduces Brain Oxidation Stress in SAMP8 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Survival Test
2.3. Experiment Design for Age-Related Cognitive Impairment
2.4. Locomotion Activity
2.5. Passive Avoidance Test
2.6. Active Avoidance Test
2.7. Oxidative Stress Analysis
2.8. Measurements of the Brain Amyloid-β Protein
2.9. Histology
2.10. Statistic
3. Results
3.1. Effect of L. plantarum GKM3 on the SAMP8 Survival Test
3.2. Body Weight, Food Intake, and Water Intake in SAMP8 Mice
3.3. Effect of L. plantarum GKM3 on Memory Retention and Learning Enhancement
3.4. Effect of L. plantarum GKM3 on Brain Oxidative Stress Reduction
3.5. Effect of L. plantarum GKM3 on Amyloid-β Participation in SAMP8 Mice Brains
3.6. Effect of L. plantarum GKM3 on Hippocampus Histology in SAMP8 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boustani, M.; Baker, M.S.; Campbell, N.; Munger, S.; Hui, S.L.; Castelluccio, P.; Farber, M.; Guzman, O.; Ademuyiwa, A.; Miller, D.; et al. Impact and recognition of cognitive impairment among hospitalized elders. J. Hosp. Med. 2010, 5, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.J.; Guerchat, M.M.; Prina, M. The Epidemiology and Impact of Dementia—Current State and Future Trends; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabda, R. An update on inflamm-aging: Mechanisms, prevention, and treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Janson, M. Orthomolecular medicine: The therapeutic use of dietary supplements for anti-aging. Clin. Interv. Aging 2006, 1, 261–265. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Tsai, M.L.; Wu, J.C.; Ho, C.T. Molecular mechanisms for anti-aging by natural dietary compounds. Mol. Nutr. Food Res. 2012, 56, 88–115. [Google Scholar] [CrossRef]
- Shin, K.K.; Yi, Y.S.; Kim, J.K.; Kim, H.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Korean red ginseng plays an anti-aging role by modulating expression of aging-related genes and immune cell subsets. Molecules 2020, 25, 1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.S.; Drieu, K.; Alm, P.; Westman, J. Role of nitric oxide in blood-brain barrier permeability, brain edema and cell damage following hyperthermic brain injury: An experimental study using EGB-761 and Gingkolide B pretreatment in the rat. Acta Neurochir. Suppl. 2000, 76, 81–86. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Lin, Q.; Liang, Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef]
- Miller, M.G.; Thangthaeng, N.; Poulose, S.M.; Shukitt-Hale, B. Role of fruits, nuts, and vegetables in maintaining cognitive health. Exp. Gerontol. 2017, 94, 24–28. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Gut microbiota and old age: Modulating factors and interventions for healthy longevity. Exp. Gerontol. 2020, 141, 111095. [Google Scholar] [CrossRef]
- Maynard, C.; Weinkove, D. Bacteria increase host micronutrient availability: Mechanisms revealed by studies in C. elegans. Genes Nutr. 2020, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Rahim, M.B.H.A.; Chilloux, J.; Martinez-Gili, L.; Neves, A.L.; Myridakis, A.; Gooderham, N.; Dumas, M.E. Diet-induced metabolic changes of the human gut microbiome: Importance of short-chain fatty acids, methylamines and indoles. Acta Diabetol. 2019, 56, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClanahan, D.; Yeh, A.; Firek, B.; Zettle, S.; Rogers, M.; Cheek, R.; Nguyen, M.V.L.; Gayer, C.P.; Wendell, S.G.; Mullet, S.J.; et al. Pilot study of the effect of plant-based enteral nutrition on the gut microbiota in chronically ill tube-fed children. J. Parenter. Enter. Nutr. 2019, 43, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.A.; Van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Singh, N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol. 2013, 13, 869–874. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, A.; Rubino, S.; Kelvin, D.J.; Carru, C. A cross-sectional study of compositional and functional profiles of gut microbiota in Sardinian centenarians. Msystems 2019, 4, e00325-19. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zinellu, A.; Milanesi, L.; Rubino, S.; Kelvin, D.J.; Carru, C. Gut Microbiota Pattern of Centenarians. In Centenarians; Springer: Berlin, Germany, 2019; pp. 149–160. [Google Scholar] [CrossRef]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2020, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, R.; Pessione, E. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunes, R.A.; Poluektova, E.U.; Vasileva, E.V.; Odorskaya, M.V.; Marsova, M.V.; Kovalev, G.I.; Danilenko, V.N. A multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with antidepressant effects. Probiotics Antimicrob. Proteins 2020, 12, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 2019, 63, 1900603. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Hammes, W.P.; Vogel, R.F. The genus lactobacillus. In The Genera of Lactic Acid Bacteria; Springer: Berlin, Germany, 1995; pp. 19–54. [Google Scholar]
- Hsu, C.L.; Hou, Y.H.; Wang, C.S.; Lin, S.W.; Jhou, B.I.; Chen, C.C.; Chen, Y.L. Antiobesity and uric acid-lowering effect of Lactobacillus plantarum GKM3 in high-fat-diet-induced obese rats. J. Am. Coll. Nutr. 2019, 38, 623–632. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Lin, S.W.; Chen, Y.L.; Chen, C.C. Effect of probiotics Lactobacillus paracasei GKS6, L. plantarum GKM3, and L. rhamnosus GKLC1 on alleviating alcohol-induced alcoholic liver disease in a mouse model. Nutr. Res. Pract. 2020, 14, 299–308. [Google Scholar] [CrossRef]
- Shih, Y.T.; Lin, S.W.; Wang, C.S.; Chen, Y.L.; Lin, W.H.; Tsai, P.C.; Chen, C.C. Effect of Probiotic Lactobacillus plantarum GKM3 on OVA-Induced Asthma in Mice. J. Financ. Quant. Anal. 2019, 8, 126–132. [Google Scholar] [CrossRef]
- Lin, W.S.; Chen, J.Y.; Wang, J.C.; Chen, L.Y.; Lin, C.H.; Hsieh, T.R.; Wang, M.F.; Fu, T.F.; Wang, P.Y. The anti-aging effects of Ludwigia octovalvis on Drosophila melanogaster and SAMP8 mice. Age 2014, 36, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Ueda, Y.; Wang, M.F.; Irei, A.V.; Sarukura, N.; Sakai, T.; Hsu, T.F. Effect of dietary lipids on longevity and memory in the SAMP8 mice. J. Nutr. Sci. Vitaminol. 2011, 57, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Román, G.; Jackson, R.E.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Hirano, T.; Balaban, E. Learning and memory. Proc. Natl. Acad. Sci. USA 2000, 97, 12403–12404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, M.C.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef]
- Sprott, R.L.; Stavnes, K.L. Active-and passive-avoidance learning in inbred mice: Transfer of training effects. Anim. Learn. Behav. 1974, 2, 225–228. [Google Scholar] [CrossRef]
- Ohta, A.; Hirano, T.; Yagi, H.; Tanaka, S.; Hosokawa, M.; Takeda, T. Behavioral characteristics of the SAM-P/8 strain in Sidman active avoidance task. Brain Res. 1989, 498, 195–198. [Google Scholar] [CrossRef]
- Branchi, I.; Ricceri, L. Active and passive avoidance. In Behavioral Genetics of the Mouse: Volume 1; Cambridge University Press: Cambridge, UK, 2013; pp. 291–298. [Google Scholar]
- Chou, M.Y.; Chen, Y.J.; Lin, L.H.; Nalao, Y.; Lin, A.L.; Wang, M.F.; Yong, S.M. Protective Effects of Hydrolyzed Chicken Extract (Probeptigen®/Cmi-168) on Memory Retention and Brain Oxidative Stress in Senescence-Accelerated Mice. Nutrients 2019, 11, 1870. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.C.; Hsu, C.K.; Wamg, M.F.; Su, T.Y. A diet containing yam reduces the cognitive deterioration and brain lipid peroxidation in mice with senescence accelerated. Int. J. Food Sci. Technol. 2004, 39, 99–107. [Google Scholar] [CrossRef]
- Peera, H.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Choi, C.W.; Shin, H.; Jin, S.P.; Bae, J.S.; Han, M.; Seo, E.Y.; Chun, J.; Chung, J.H. Comparison of the gut microbiota of centenarians in longevity villages of South Korea with those of other age groups. Korean J. Microbiol. Biotechnol. 2019, 29, 429–440. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Fibt, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert. Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Hou, Q.; Wang, Y.; Shen, L.; Sun, Z.; Zhang, H.; Liong, M.; Kwok, L. Long-term administration of Lactobacillus casei Zhang stabilized gut microbiota of adults and reduced gut microbiota age index of older adults. J. Funct. Foods. 2020, 64, 103682. [Google Scholar] [CrossRef]
- Bagga, D.; Reichert, J.L.; Koschuting, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schöpf, V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018, 9, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Tooley, K.L. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef] [PubMed]
- Akiguchi, I.; Pallàs, P.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 2017, 37, 293–305. [Google Scholar] [CrossRef] [Green Version]
- del Valle, J.; Duran-Vilaregut, J.; Manich, G.; Casadesús, G.; Smith, A.M.; Camins, A.; Pallàs, M.; Pelegrí, C.; Vilaplana, J. Early amyloid accumulation in the hippocampus of SAMP8 mice. J. Alzheimer’s Dis. 2010, 19, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Allan Butterfield, D. Amyloid β-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Tu, X.; Shen, H.; Qiu, H.; Chen, H.; He, J. High serum levels of malondialdehyde and 8-OHdG are both associated with early cognitive impairment in patients with acute ischaemic stroke. Sci. Rep. 2017, 7, 9493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, A.R.; Moore, M.J.; Bloss, E.B.; Mensh, B.D.; Kath, W.L.; Spruston, N. Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 2012, 76, 776–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.Z.; Zhao, Y.; Chen, H.Z. MicroRNA-98 reduces amyloid β-protein production and improves oxidative stress and mitochondrial dysfunction through the Notch signaling pathway via HEY2 in Alzheimer’s disease mice. Int. J. Mol. Med. 2019, 43, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sex | Group | Body Weight (g) | Food Intake (g/day) | Water Consumption (mL/day) | ||
|---|---|---|---|---|---|---|
| Initial (Week 0) | Final (Week 14th) | Gain | ||||
| Male | Control | 28.37 ± 1.03 a | 32.06 ± 1.05 b | 3.69 ± 0.47 c | 4.79 ± 0.06 d | 6.67 ± 0.10 e |
| GKM3-L | 28.81 ± 0.78 a | 31.14 ± 1.01 b | 2.33 ± 0.36 c | 4.74 ± 0.07 d | 6.65 ± 0.16 e | |
| GKM3-H | 28.58 ± 0.76 a | 32.12 ± 1.03 b | 3.55 ± 0.57 c | 4.71 ± 0.08 d | 6.53 ± 0.15 e | |
| Female | Control | 27.14 ± 0.95 f | 27.61 ± 0.87 g | 0.47 ± 0.61 h | 4.09 ± 0.04 i | 4.64 ± 0.14 j |
| GKM3-L | 27.01 ± 0.73 f | 28.05 ± 0.85 g | 1.49 ± 0.70 h | 3.98 ± 0.11 i | 4.60 ± 0.06 j | |
| GKM3-H | 26.63 ± 0.39 f | 28.51 ± 0.84 g | 1.88 ± 1.02 h | 4.10 ± 0.14 i | 4.69 ± 0.07 j | |
| Sex | Group | Locomotion Time Interval (Minutes)2 | |
|---|---|---|---|
| 0–5 (s/5 min) | 6–10 (s/5 min) | ||
| Male | Control | 94.90 ± 7.91 a | 86.40 ± 5.89 a |
| GKM3-L | 111.10 ± 4.95 a | 84.80 ± 6.36 a | |
| GKM3-H | 92.70 ± 2.93 a | 80.20 ± 3.74 a | |
| Female | Control | 85.10 ± 8.24 a | 84.10 ± 6.12 a |
| GKM3-L | 88.10 ± 4.79 a | 72.10 ± 6.93 a | |
| GKM3-H | 121.20 ± 16.25 a | 117.70 ± 6.93 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-W.; Tsai, Y.-S.; Chen, Y.-L.; Wang, M.-F.; Chen, C.-C.; Lin, W.-H.; Fang, T.J. Lactobacillus plantarum GKM3 Promotes Longevity, Memory Retention, and Reduces Brain Oxidation Stress in SAMP8 Mice. Nutrients 2021, 13, 2860. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082860
Lin S-W, Tsai Y-S, Chen Y-L, Wang M-F, Chen C-C, Lin W-H, Fang TJ. Lactobacillus plantarum GKM3 Promotes Longevity, Memory Retention, and Reduces Brain Oxidation Stress in SAMP8 Mice. Nutrients. 2021; 13(8):2860. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082860
Chicago/Turabian StyleLin, Shih-Wei, You-Shan Tsai, Yen-Lien Chen, Ming-Fu Wang, Chin-Chu Chen, Wen-Hsin Lin, and Tony J. Fang. 2021. "Lactobacillus plantarum GKM3 Promotes Longevity, Memory Retention, and Reduces Brain Oxidation Stress in SAMP8 Mice" Nutrients 13, no. 8: 2860. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13082860