Maternal DHA Supplementation during Pregnancy and Lactation in the Rat Protects the Offspring against High-Calorie Diet-Induced Hepatic Steatosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Nutritional Interventions
2.2. Measurement of Body Weight and Food Intake
2.3. Collection of Biological Samples and Euthanasia
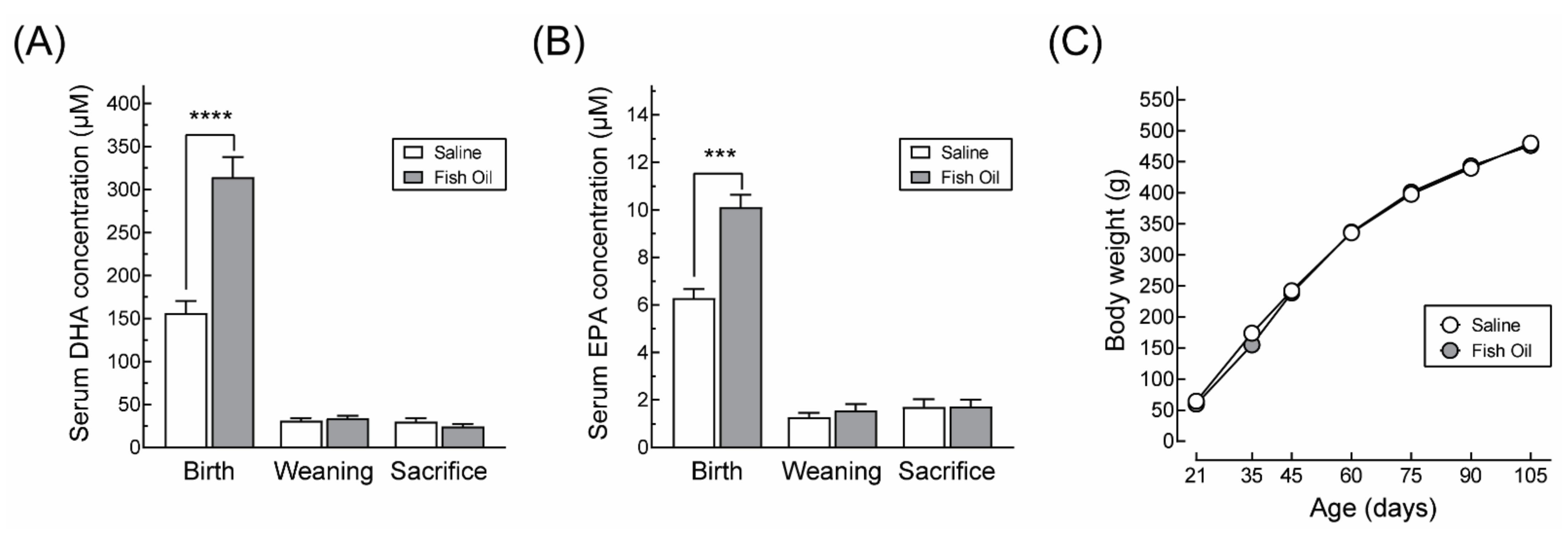
2.4. Quantification of DHA and EPA
2.5. Serum Metabolite Determinations
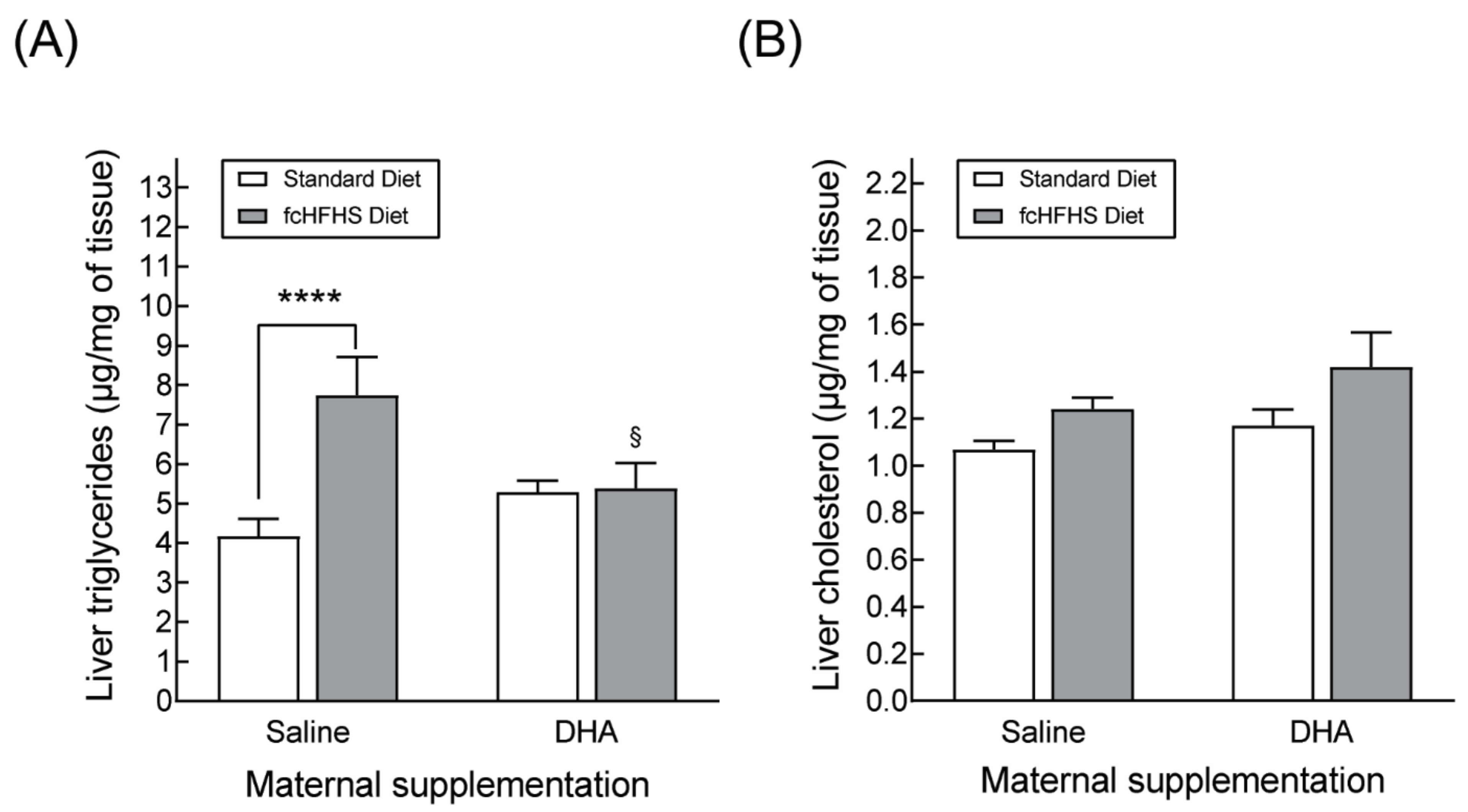
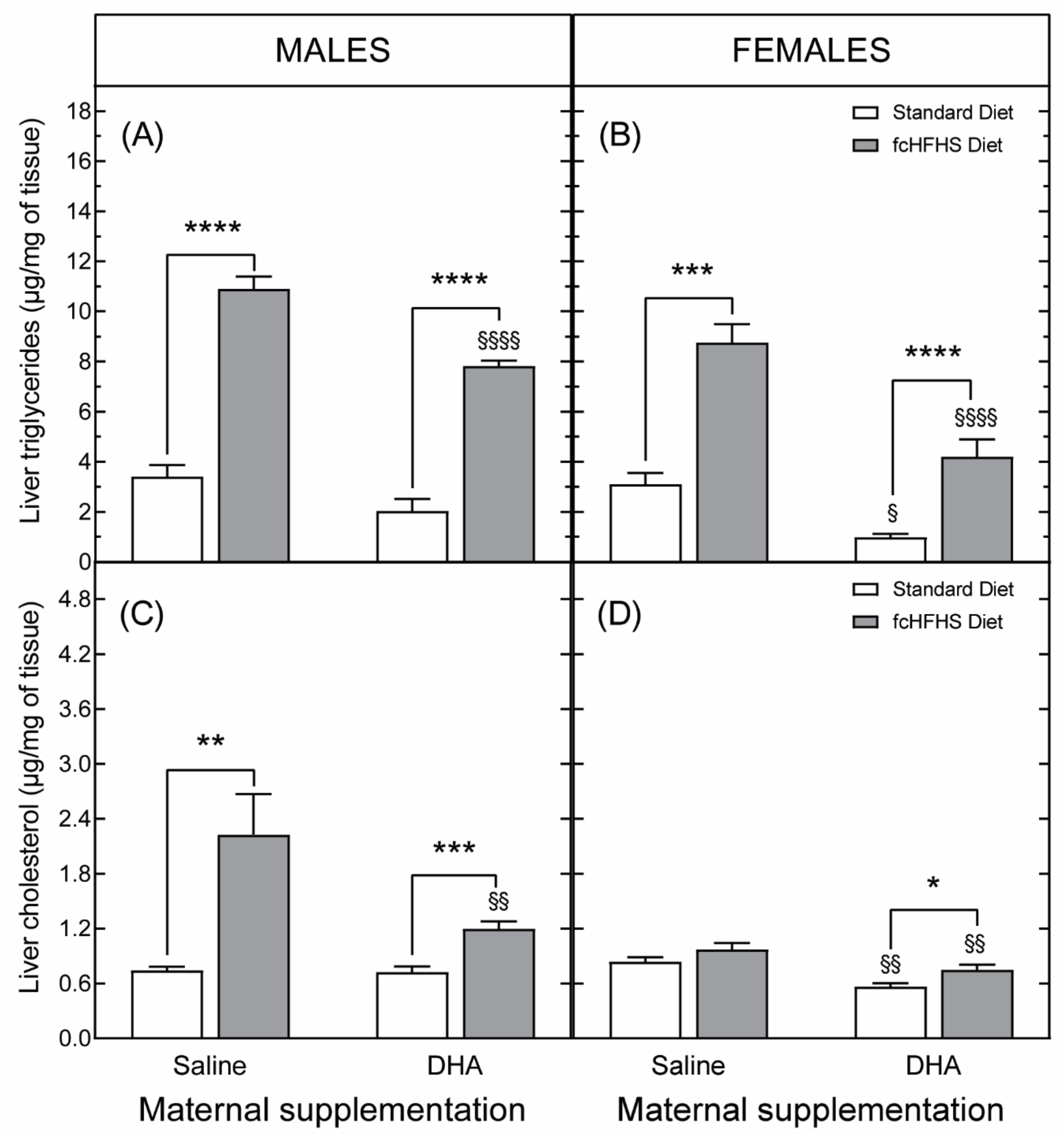
2.6. Quantification of Liver Triglycerides and Cholesterol
2.7. Real-Time Quantitative RT-PCR Experiments
2.8. Data Analysis
3. Results
3.1. DHA Supplementation Does Not Affect Maternal Body Weight Gain or Food Intake during Pregnancy nor the Offspring’s Growth Profile
3.2. Impact of Maternal DHA Supplementation on Male Offspring at Three Months
3.2.1. Anthropometric Characteristics and Serum Metabolite Profile
3.2.2. Hepatic Lipid Accumulation
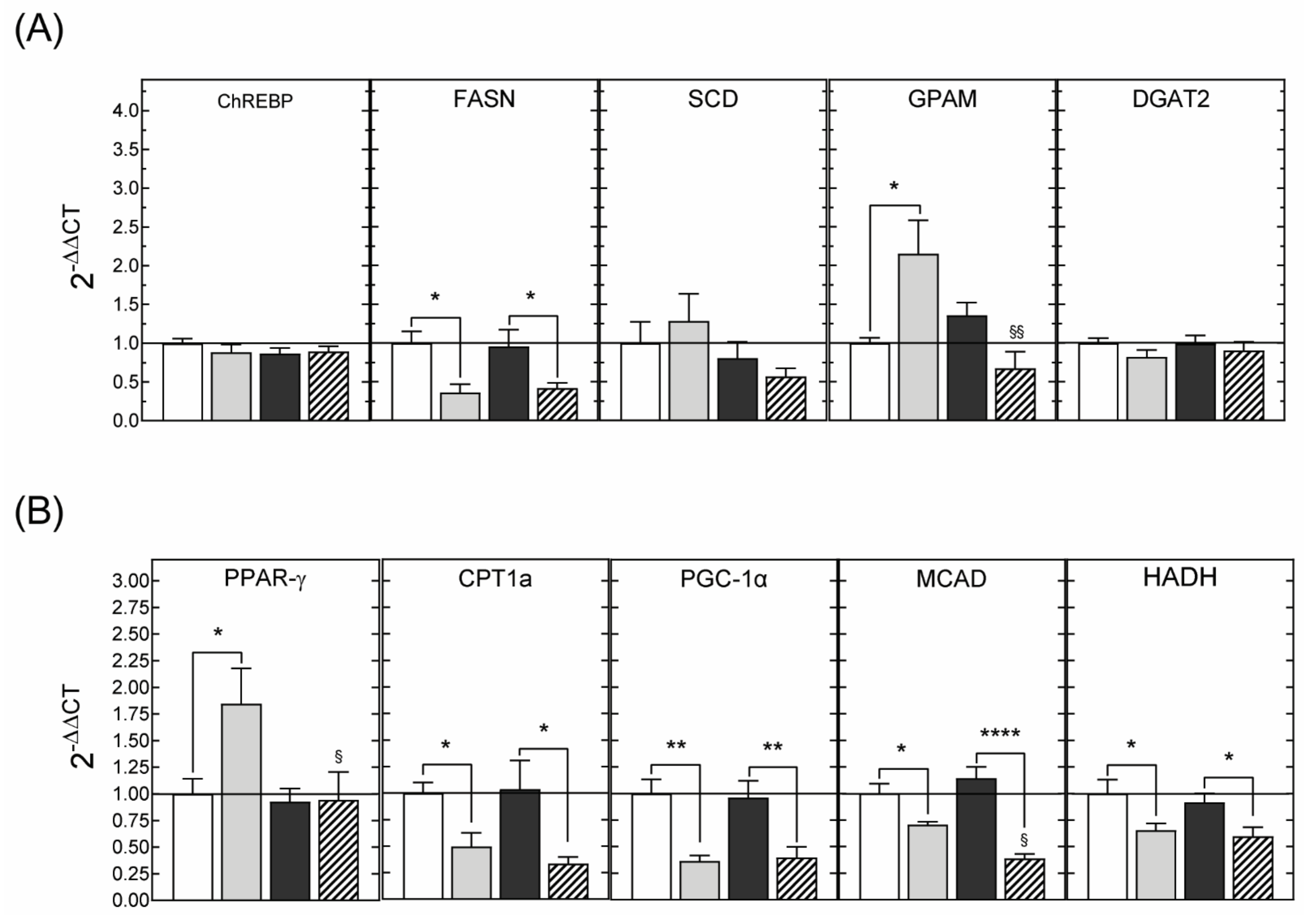
3.2.3. Hepatic Gene Expression
3.3. Impact of Maternal DHA Supplementation on the Offspring at Six Months
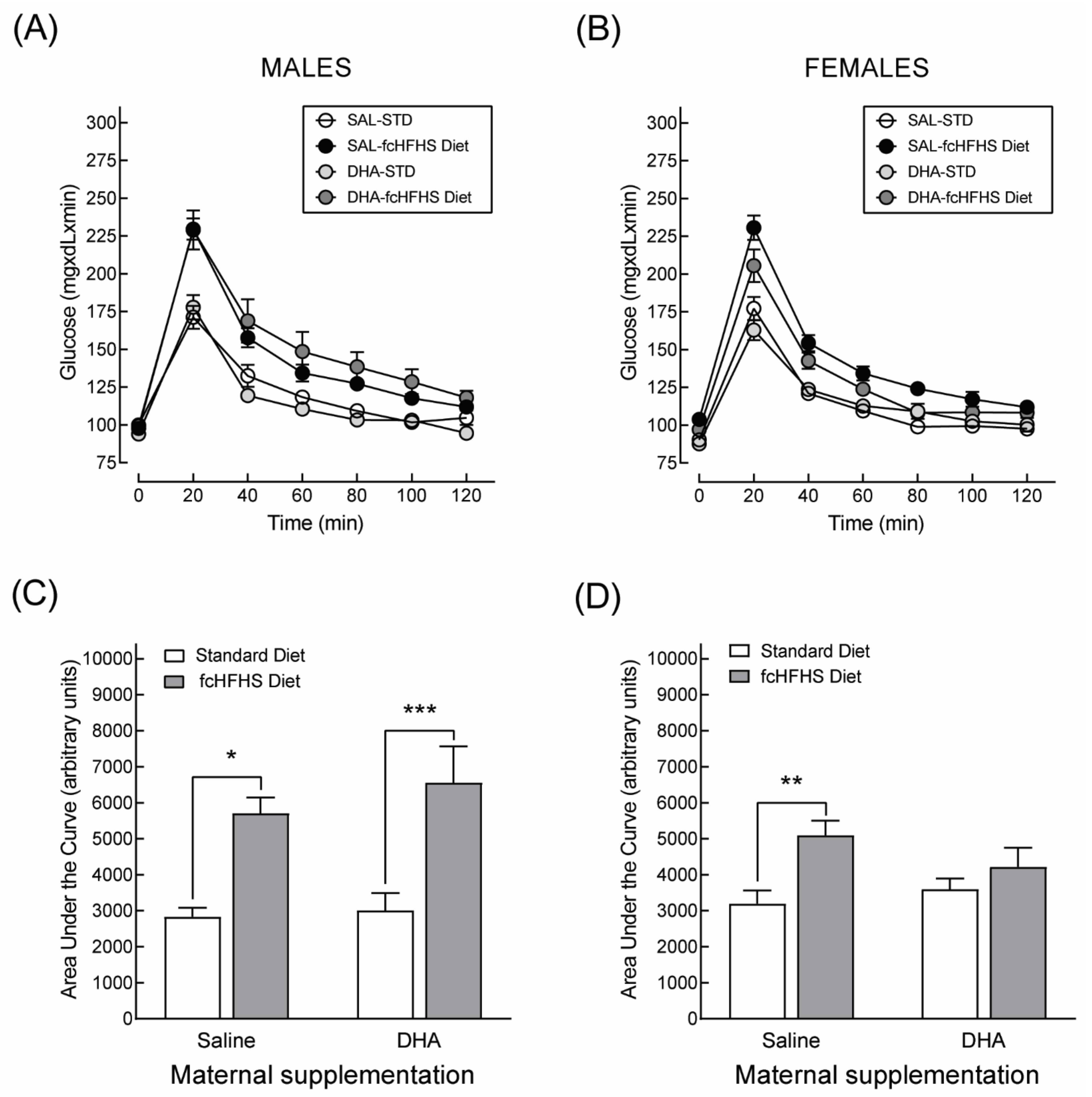
3.3.1. Anthropometric Characteristics and Serum Metabolite Profile
3.3.2. Hepatic Liver Accumulation
3.3.3. Hepatic Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of Maternal Obesity on the Long-Term Health of Offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and Child Undernutrition Study Group Maternal and Child Undernutrition: Consequences for Adult Health and Human Capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef] [Green Version]
- Edlow, A.G. Maternal Obesity and Neurodevelopmental and Psychiatric Disorders in Offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Correia-Branco, A.; Keating, E.; Martel, F. Maternal Undernutrition and Fetal Developmental Programming of Obesity: The Glucocorticoid Connection. Reprod. Sci. 2015, 22, 138–145. [Google Scholar] [CrossRef]
- Barker, D.J.P. The Developmental Origins of Chronic Adult Disease. Acta Paediatr. Suppl. 2004, 93, 26–33. [Google Scholar] [CrossRef]
- Symonds, M.E.; Sebert, S.P.; Hyatt, M.A.; Budge, H. Nutritional Programming of the Metabolic Syndrome. Nat. Rev. Endocrinol. 2009, 5, 604–610. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Hjort, L.; Novakovic, B.; Ozanne, S.E.; Saffery, R. Intrauterine Programming of Obesity and Type 2 Diabetes. Diabetologia 2019, 62, 1789–1801. [Google Scholar] [CrossRef] [Green Version]
- Castro-Rodríguez, D.C.; Rodríguez-González, G.L.; Menjivar, M.; Zambrano, E. Maternal Interventions to Prevent Adverse Fetal Programming Outcomes Due to Maternal Malnutrition: Evidence in Animal Models. Placenta 2020, 102, 49–54. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Tain, Y.-L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The Roles of Long-Chain Polyunsaturated Fatty Acids in Pregnancy, Lactation and Infancy: Review of Current Knowledge and Consensus Recommendations. J. Perinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.-C.; Tung, C.-Y.; Chen, H.-E. Omega-3 Polyunsaturated Fatty Acid Supplementation in Prevention and Treatment of Maternal Depression: Putative Mechanism and Recommendation. J. Affect Disord. 2018, 238, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Guu, T.-W.; Mischoulon, D.; Sarris, J.; Hibbeln, J.; McNamara, R.K.; Hamazaki, K.; Freeman, M.P.; Maes, M.; Matsuoka, Y.J.; Belmaker, R.H.; et al. International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder. Psychother. Psychosom. 2019, 88, 263–273. [Google Scholar] [CrossRef]
- Carlson, S.E.; Gajewski, B.J.; Alhayek, S.; Colombo, J.; Kerling, E.H.; Gustafson, K.M. Dose-Response Relationship between Docosahexaenoic Acid (DHA) Intake and Lower Rates of Early Preterm Birth, Low Birth Weight and Very Low Birth Weight. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Astwood, J.D.; Gautier, S.; Kuratko, C.N.; Nelson, E.B.; Salem, N. Effects of Long-Chain Polyunsaturated Fatty Acid Supplementation on Neurodevelopment in Childhood: A Review of Human Studies. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 305–314. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Stein, A.D.; Parra-Cabrera, S.; Wang, M.; Imhoff-Kunsch, B.; Juárez-Márquez, S.; Rivera, J.; Martorell, R. Effects of Docosahexaenoic Acid Supplementation during Pregnancy on Gestational Age and Size at Birth: Randomized, Double-Blind, Placebo-Controlled Trial in Mexico. Food Nutr. Bull. 2010, 31, S108–S116. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P. DOMInO Investigative Team Effect of DHA Supplementation during Pregnancy on Maternal Depression and Neurodevelopment of Young Children: A Randomized Controlled Trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef] [Green Version]
- Couet, C.; Delarue, J.; Ritz, P.; Antoine, J.M.; Lamisse, F. Effect of Dietary Fish Oil on Body Fat Mass and Basal Fat Oxidation in Healthy Adults. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Kabir, M.; Skurnik, G.; Naour, N.; Pechtner, V.; Meugnier, E.; Rome, S.; Quignard-Boulangé, A.; Vidal, H.; Slama, G.; Clément, K.; et al. Treatment for 2 Mo with n 3 Polyunsaturated Fatty Acids Reduces Adiposity and Some Atherogenic Factors but Does Not Improve Insulin Sensitivity in Women with Type 2 Diabetes: A Randomized Controlled Study. Am. J. Clin. Nutr. 2007, 86, 1670–1679. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The Clinical Relevance of Omega-3 Fatty Acids in the Management of Hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef] [Green Version]
- Spooner, M.H.; Jump, D.B. Omega-3 Fatty Acids and Nonalcoholic Fatty Liver Disease in Adults and Children: Where Do We Stand? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zahradka, P.; Cordero-Monroy, L.; Wright, B.; Taylor, C.G. Dietary Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) Operate by Different Mechanisms to Modulate Hepatic Steatosis and Hyperinsulemia in Fa/Fa Zucker Rats. Nutrients 2019, 11, 917. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.S.; Bulchandani, D. Why Do Omega-3 Fatty Acids Lower Serum Triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef]
- Shearer, G.C.; Savinova, O.V.; Harris, W.S. Fish Oil—How Does It Reduce Plasma Triglycerides? Biochim. Biophys. Acta 2012, 1821, 843–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oscarsson, J.; Hurt-Camejo, E. Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Their Mechanisms of Action on Apolipoprotein B-Containing Lipoproteins in Humans: A Review. Lipids Health Dis. 2017, 16, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.J.; Pramfalk, C.; Charlton, C.A.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de Novo Lipogenesis Is Suppressed and Fat Oxidation Is Increased by Omega-3 Fatty Acids at the Expense of Glucose Metabolism. BMJ Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef] [Green Version]
- Grevengoed, T.J.; Trammell, S.A.; Svenningsen, J.S.; Makarov, M.V.; Nielsen, T.S.; Jacobsen, J.C.B.; Treebak, J.T.; Calder, P.C.; Migaud, M.E.; Cravatt, B.F.; et al. An Abundant Biliary Metabolite Derived from Dietary Omega-3 Polyunsaturated Fatty Acids Regulates Triglycerides. J. Clin. Investig. 2021, 131, 143861. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.K.; Ward, L.C.; Brown, L. Effects of ALA, EPA and DHA in High-Carbohydrate, High-Fat Diet-Induced Metabolic Syndrome in Rats. J. Nutr. Biochem. 2013, 24, 1041–1052. [Google Scholar] [CrossRef]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of Marine Origin Limit Diet-Induced Obesity in Mice by Reducing Cellularity of Adipose Tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef]
- La Fleur, S.E.; van Rozen, A.J.; Luijendijk, M.C.M.; Groeneweg, F.; Adan, R.a.H. A Free-Choice High-Fat High-Sugar Diet Induces Changes in Arcuate Neuropeptide Expression That Support Hyperphagia. Int. J. Obes. 2010, 34, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Sherman, R.C.; Ali, Z.; Wootton, S.A.; Jackson, A.A. Docosahexaenoic Acid Is Selectively Enriched in Plasma Phospholipids during Pregnancy in Trinidadian Women--Results of a Pilot Study. Reprod. Nutr. Dev. 2006, 46, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, F.; Rodie, V.A.; Ramsay, J.E.; Greer, I.A.; Freeman, D.J.; Meyer, B.J. Longitudinal Assessment of Erythrocyte Fatty Acid Composition throughout Pregnancy and Post Partum. Lipids 2007, 42, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.E.; Hoile, S.P.; Burdge, G.C.; Calder, P.C. Changes in Rat N-3 and n-6 Fatty Acid Composition during Pregnancy Are Associated with Progesterone Concentrations and Hepatic FADS2 Expression. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Yang, J.; Cunnane, S.C. Gestational Hyperlipidemia in the Rat Is Characterized by Accumulation of n −6 and n −3 Fatty Acids, Especially Docosahexaenoic Acid. Biochim. Biophys. Acta 1992, 1127, 263–269. [Google Scholar] [CrossRef]
- Luo, X.; He, Z.; Sun, X.; Gu, X.; Zhang, W.; Gao, J.; Li, X.; Jia, R.; Wei, J.; Yu, Y.; et al. DHA Protects Against Hepatic Steatosis by Activating Sirt1 in a High Fat Diet-Induced Nonalcoholic Fatty Liver Disease Mouse Model. Diabetes Metab. Syndr. Obes. 2020, 13, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, R.; Videla, L.A. Impact of the Co-Administration of N-3 Fatty Acids and Olive Oil Components in Preclinical Nonalcoholic Fatty Liver Disease Models: A Mechanistic View. Nutrients 2020, 12, 499. [Google Scholar] [CrossRef] [Green Version]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [Green Version]
- Bradberry, J.C.; Hilleman, D.E. Overview of Omega-3 Fatty Acid Therapies. Pharm. Ther. 2013, 38, 681–691. [Google Scholar]
- Dangardt, F.; Chen, Y.; Gronowitz, E.; Dahlgren, J.; Friberg, P.; Strandvik, B. High Physiological Omega-3 Fatty Acid Supplementation Affects Muscle Fatty Acid Composition and Glucose and Insulin Homeostasis in Obese Adolescents. J. Nutr. Metab. 2012, 2012, 395757. [Google Scholar] [CrossRef] [Green Version]
- Storlien, L.H.; Kraegen, E.W.; Chisholm, D.J.; Ford, G.L.; Bruce, D.G.; Pascoe, W.S. Fish Oil Prevents Insulin Resistance Induced by High-Fat Feeding in Rats. Science 1987, 237, 885–888. [Google Scholar] [CrossRef]
- Vahdaninia, M.; Mackenzie, H.; Dean, T.; Helps, S. The Effectiveness of ω-3 Polyunsaturated Fatty Acid Interventions during Pregnancy on Obesity Measures in the Offspring: An up-to-Date Systematic Review and Meta-Analysis. Eur. J. Nutr. 2019, 58, 2597–2613. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Casanova, I.; Stein, A.D.; Hao, W.; Garcia-Feregrino, R.; Barraza-Villarreal, A.; Romieu, I.; Rivera, J.A.; Martorell, R.; Ramakrishnan, U. Prenatal Supplementation with Docosahexaenoic Acid Has No Effect on Growth through 60 Months of Age. J. Nutr. 2015, 145, 1330–1334. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Gomez, Y.; Stein, A.D.; Ramakrishnan, U.; Barraza-Villarreal, A.; Moreno-Macias, H.; Aguilar-Salinas, C.; Romieu, I.; Rivera, J.A. Prenatal Docosahexaenoic Acid Supplementation Does Not Affect Nonfasting Serum Lipid and Glucose Concentrations of Offspring at 4 Years of Age in a Follow-Up of a Randomized Controlled Clinical Trial in Mexico. J. Nutr. 2017, 147, 242–247. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Miljkovic, D.; Fong, L.; Xian, C.J.; Duthoit, E.; Gibson, R.A. Maternal Omega-3 Supplementation Increases Fat Mass in Male and Female Rat Offspring. Front. Genet. 2011, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Vidakovic, A.J.; Gishti, O.; Voortman, T.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Jaddoe, V.W.V.; et al. Maternal Plasma PUFA Concentrations during Pregnancy and Childhood Adiposity: The Generation R Study. Am. J. Clin. Nutr. 2016, 103, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Donahue, S.M.A.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal Fatty Acid Status and Child Adiposity at Age 3 y: Results from a US Pregnancy Cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Casanova, I.; Rzehak, P.; Stein, A.D.; Garcia Feregrino, R.; Rivera Dommarco, J.A.; Barraza-Villarreal, A.; Demmelmair, H.; Romieu, I.; Villalpando, S.; Martorell, R.; et al. Maternal Single Nucleotide Polymorphisms in the Fatty Acid Desaturase 1 and 2 Coding Regions Modify the Impact of Prenatal Supplementation with DHA on Birth Weight. Am. J. Clin. Nutr. 2016, 103, 1171–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Fleur, S.E.; Luijendijk, M.C.M.; van Rozen, A.J.; Kalsbeek, A.; Adan, R.a.H. A Free-Choice High-Fat High-Sugar Diet Induces Glucose Intolerance and Insulin Unresponsiveness to a Glucose Load Not Explained by Obesity. Int. J. Obes. 2011, 35, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diepenbroek, C.; Eggels, L.; Ackermans, M.T.; Fliers, E.; Kalsbeek, A.; Serlie, M.J.; la Fleur, S.E. Differential Effects of Hypercaloric Choice Diets on Insulin Sensitivity in Rats. J. Endocrinol. 2017, 232, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Buijs, R.M. A Suprachiasmatic Nucleus Generated Rhythm in Basal Glucose Concentrations. J. Neuroendocr. 1999, 11, 643–652. [Google Scholar] [CrossRef]
- Bhaswant, M.; Poudyal, H.; Brown, L. Mechanisms of Enhanced Insulin Secretion and Sensitivity with N-3 Unsaturated Fatty Acids. J. Nutr. Biochem. 2015, 26, 571–584. [Google Scholar] [CrossRef]
- Bai, T.; Yang, H.; Wang, H.; Zhi, L.; Liu, T.; Cui, L.; Liu, W.; Wang, Y.; Zhang, M.; Liu, Y.; et al. Inhibition of Voltage-Gated K± Channels Mediates Docosahexaenoic Acid-Stimulated Insulin Secretion in Rat Pancreatic β-Cells. Food Funct. 2020, 11, 8893–8904. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and Disease Consequences of Nonalcoholic Fatty Liver Disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 Fatty Acids, Hepatic Lipid Metabolism, and Nonalcoholic Fatty Liver Disease. Annu. Rev. Nutr. 2013, 33, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Lee, K.-T.; Park, Y.B.; Jeon, S.-M.; Choi, M.-S. Dietary Docosahexaenoic Acid-Rich Diacylglycerols Ameliorate Hepatic Steatosis and Alter Hepatic Gene Expressions in C57BL/6J-Lep(Ob/Ob) Mice. Mol. Nutr. Food Res. 2008, 52, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; et al. Re-Evaluation of Fatty Acid Metabolism-Related Gene Expression in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasarathy, S.; Kasumov, T.; Edmison, J.M.; Gruca, L.L.; Bennett, C.; Duenas, C.; Marczewski, S.; McCullough, A.J.; Hanson, R.W.; Kalhan, S.C. Glycine and Urea Kinetics in Nonalcoholic Steatohepatitis in Human: Effect of Intralipid Infusion. Am. J. Physiol. Gastrointest Liver Physiol 2009, 297, G567–G575. [Google Scholar] [CrossRef] [Green Version]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin Resistance in Non-Diabetic Patients with Non-Alcoholic Fatty Liver Disease: Sites and Mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Croci, I.; Byrne, N.M.; Choquette, S.; Hills, A.P.; Chachay, V.S.; Clouston, A.D.; O’Moore-Sullivan, T.M.; Macdonald, G.A.; Prins, J.B.; Hickman, I.J. Whole-Body Substrate Metabolism Is Associated with Disease Severity in Patients with Non-Alcoholic Fatty Liver Disease. Gut 2013, 62, 1625–1633. [Google Scholar] [CrossRef] [Green Version]
- Kotronen, A.; Seppälä-Lindroos, A.; Vehkavaara, S.; Bergholm, R.; Frayn, K.N.; Fielding, B.A.; Yki-Järvinen, H. Liver Fat and Lipid Oxidation in Humans. Liver Int. 2009, 29, 1439–1446. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [Green Version]
- Madsen, L.; Rustan, A.C.; Vaagenes, H.; Berge, K.; Dyrøy, E.; Berge, R.K. Eicosapentaenoic and Docosahexaenoic Acid Affect Mitochondrial and Peroxisomal Fatty Acid Oxidation in Relation to Substrate Preference. Lipids 1999, 34, 951–963. [Google Scholar] [CrossRef]
- Suzuki-Kemuriyama, N.; Matsuzaka, T.; Kuba, M.; Ohno, H.; Han, S.; Takeuchi, Y.; Isaka, M.; Kobayashi, K.; Iwasaki, H.; Yatoh, S.; et al. Different Effects of Eicosapentaenoic and Docosahexaenoic Acids on Atherogenic High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE 2016, 11, e0157580. [Google Scholar] [CrossRef] [Green Version]
- Rillamas-Sun, E.; LaCroix, A.Z.; Waring, M.E.; Kroenke, C.H.; LaMonte, M.J.; Vitolins, M.Z.; Seguin, R.; Bell, C.L.; Gass, M.; Manini, T.M.; et al. Obesity and Late-Age Survival without Major Disease or Disability in Older Women. JAMA Intern. Med. 2014, 174, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Tucsek, Z.; Toth, P.; Tarantini, S.; Sosnowska, D.; Gautam, T.; Warrington, J.P.; Giles, C.B.; Wren, J.D.; Koller, A.; Ballabh, P.; et al. Aging Exacerbates Obesity-Induced Cerebromicrovascular Rarefaction, Neurovascular Uncoupling, and Cognitive Decline in Mice. J. Gerontol. Ser. A 2014, 69, 1339–1352. [Google Scholar] [CrossRef]
- Zore, T.; Palafox, M.; Reue, K. Sex Differences in Obesity, Lipid Metabolism, and Inflammation-A Role for the Sex Chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef]
- Link, J.C.; Reue, K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu. Rev. Nutr. 2017, 37, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Casellas, A.; Proenza, A.M.; Lladó, I.; Gianotti, M. Sex-Dependent Differences in Rat Hepatic Lipid Accumulation and Insulin Sensitivity in Response to Diet-Induced Obesity. Biochem. Cell Biol. 2012, 90, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.J.; Hecht, P.M.; Jasarevic, E.; Beversdorf, D.Q.; Will, M.J.; Fritsche, K.; Gillespie, C.H. Sex-Specific Effects of Docosahexaenoic Acid (DHA) on the Microbiome and Behavior of Socially-Isolated Mice. Brain Behav. Immun. 2017, 59, 38–48. [Google Scholar] [CrossRef]
- Mendonça, A.M.; Cayer, L.G.J.; Pauls, S.D.; Winter, T.; Leng, S.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Distinct Effects of Dietary ALA, EPA and DHA on Rat Adipose Oxylipins Vary by Depot Location and Sex. Prostaglandins Leukot. Essent. Fat. Acids 2018, 129, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.-F.; Montagner, A.; Gourdy, P. Sex Differences in Metabolic Regulation and Diabetes Susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E. Sex Differences in the Control of Glucose Homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Kasbi-Chadli, F.; Ferchaud-Roucher, V.; Krempf, M.; Ouguerram, K. Direct and Maternal N-3 Long-Chain Polyunsaturated Fatty Acid Supplementation Improved Triglyceridemia and Glycemia through the Regulation of Hepatic and Muscle Sphingolipid Synthesis in Offspring Hamsters Fed a High-Fat Diet. Eur. J. Nutr. 2016, 55, 589–599. [Google Scholar] [CrossRef]
- Kasbi-Chadli, F.; Boquien, C.-Y.; Simard, G.; Ulmann, L.; Mimouni, V.; Leray, V.; Meynier, A.; Ferchaud-Roucher, V.; Champ, M.; Nguyen, P.; et al. Maternal Supplementation with N-3 Long Chain Polyunsaturated Fatty Acids during Perinatal Period Alleviates the Metabolic Syndrome Disturbances in Adult Hamster Pups Fed a High-Fat Diet after Weaning. J. Nutr. Biochem. 2014, 25, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wei, Z.; Li, Y. DHA Regulates Lipogenesis and Lipolysis Genes in Mice Adipose and Liver. Mol. Biol. Rep. 2011, 38, 731–737. [Google Scholar] [CrossRef]
- Okada, L.S.D.R.R.; Oliveira, C.P.; Stefano, J.T.; Nogueira, M.A.; da Silva, I.D.C.G.; Cordeiro, F.B.; Alves, V.A.F.; Torrinhas, R.S.; Carrilho, F.J.; Puri, P.; et al. Omega-3 PUFA Modulate Lipogenesis, ER Stress, and Mitochondrial Dysfunction Markers in NASH—Proteomic and Lipidomic Insight. Clin. Nutr. 2018, 37, 1474–1484. [Google Scholar] [CrossRef]
- Lee, H.-S.; Barraza-Villarreal, A.; Biessy, C.; Duarte-Salles, T.; Sly, P.D.; Ramakrishnan, U.; Rivera, J.; Herceg, Z.; Romieu, I. Dietary Supplementation with Polyunsaturated Fatty Acid during Pregnancy Modulates DNA Methylation at IGF2/H19 Imprinted Genes and Growth of Infants. Physiol. Genom. 2014, 46, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, S.J.; Zhou, J.; Peters, T.J.; Buckley, M.; Sutcliffe, B.; Oytam, Y.; Gibson, R.A.; McPhee, A.; Yelland, L.N.; Makrides, M.; et al. Effect of Prenatal DHA Supplementation on the Infant Epigenome: Results from a Randomized Controlled Trial. Clin. Epigenetics 2016, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Amarasekera, M.; Noakes, P.; Strickland, D.; Saffery, R.; Martino, D.J.; Prescott, S.L. Epigenome-Wide Analysis of Neonatal CD4(±) T-Cell DNA Methylation Sites Potentially Affected by Maternal Fish Oil Supplementation. Epigenetics 2014, 9, 1570–1576. [Google Scholar] [CrossRef] [Green Version]
- Joss-Moore, L.A.; Wang, Y.; Baack, M.L.; Yao, J.; Norris, A.W.; Yu, X.; Callaway, C.W.; McKnight, R.A.; Albertine, K.H.; Lane, R.H. IUGR Decreases PPARγ and SETD8 Expression in Neonatal Rat Lung and These Effects Are Ameliorated by Maternal DHA Supplementation. Early Hum. Dev. 2010, 86, 785–791. [Google Scholar] [CrossRef] [Green Version]





| Gene Name | Symbol | Forward Primer | Reverse Primer |
|---|---|---|---|
| Carbohydrate response element binding protein | ChREBP | GTACTGTTCCCTGCCTGCTCTC | CCCTCTGTGACTGCCCTTGTG |
| Fatty acid synthase | FASN | CGCCGTGGTGCTGGAGATTG | CTTGCCGAGGTTGGTGAGGAAG |
| Stearoyl-coenzyme A desaturase | SCD | GGAGCCACAGGACTTACAAGG | CGCACAAGCAGCCAACCC |
| Glycerol-3-phosphate acyltransferase, mitochondrial | GPAM | TGGAGTGTGGCAAGAGGCGTTATC | TTCGGCAGCAGCAGCATCAGG |
| Diacylglycerol O-acyltransferase 2 | DGAT2 | CCTCATCGCTGCCTACTCC | TGAGCCAGGTGACAGAGAAG |
| Peroxisome proliferator-activated receptor delta | PPARγ | GGAATTAGATGACAGTGACTTGGC | GGAGCACCTTGGCGAACAG |
| Carnitine palmitoyltransferase 1A | CPT1a | TGCCTGCCAGTTCCATTAAGC | GTCTCACTCCTCTTGCCAACAG |
| Peroxisome proliferator-activated receptor gamma, co-activator 1alpha | PGC-1α | ACACCGCACACATCGCAATTC | TTCGTCCCTCTTGAGCCTTTCG |
| Medium chain acyl coenzyme A dehydrogenase | MCAD | GAGGCTAGAAGGTCCTGAGAAGTG | TCTGCTGCTCCGTCAACTGG |
| Hydroxyacyl-Coenzyme A dehydrogenase | HADH | CTCCATGTCCTCCTCTTCCTCTGC | CAGCCCGCCGCCGATGAC |
| 18S ribosomal RNA | 18S | GATGCGGCGGCGTTATTCC | CTCCTGGTGGTGCCCTTCC |
| Beta-2 microglobulin | β2M | GATGGCTCGCTCGGTGAC | CGTAGCAGTTGAGGAAGTTGG |
| Supplemented Saline | Supplemented DHA | |||
|---|---|---|---|---|
| Standard Diet | FcHFHS Diet | Standard Diet | FcHFHS Diet | |
| Body weight (g) | 510 ± 12 | 600 ± 16 **** | 477 ± 10 | 599 ± 15 **** |
| Adipose Index | 4.40 ± 0.20 | 7.36 ± 0.31 **** | 4.76 ± 0.23 | 6.66 ± 0.32 *** |
| Leptin (ng/mL) | 12.43 ± 0.83 | 33.10 ± 4.31 **** | 13.72 ± 2.29 | 34.58 ± 5.44 ** |
| Triglycerides (mg/dL) | 95.48 ± 10.45 | 157.49 ± 15.70 ** | 91.27 ± 5.11 | 171.58 ± 28.29 * |
| Cholesterol (mg/dL) | 67.54 ± 3.54 | 77.24 ± 2.96 | 64.72 ± 4.64 | 72.38 ± 4.32 |
| Glucose (mg/dL) | 189.54 ± 5.43 | 206.66 ± 6.27 | 197.20 ± 8.26 | 204.16 ± 9.50 |
| MALES | ||||
|---|---|---|---|---|
| Supplemented Saline | Supplemented DHA | |||
| Standard Diet | fcHFHS Diet | Standard Diet | FcHFHS Diet | |
| Body weight (g) | 633 ± 19 | 754 ± 24 *** | 630 ± 20 | 770 ± 24 *** |
| Adipose Index | 6.51 ± 0.43 | 9.79 ± 0.37 **** | 7.05 ± 0.11 | 10.72 ± 0.85 *** |
| Leptin (ng/mL) | 14.99 ± 1.76 | 26.04 ± 1.91 ** | 15.69 ± 1.37 | 30.27 ± 3.55 *** |
| Triglycerides (mg/dL) | 101.44 ± 10.96 | 151.09± 20.08 * | 65.61 ± 7.49 | 119.45± 18.16 * |
| Cholesterol (mg/dL) | 73.62 ± 5.54 | 74.83 ± 2.90 | 79.80 ± 6.11 | 72.94 ± 2.85 |
| Glucose (mg/dL) | 155.24 ± 9.04 | 177.42 ± 22.61 | 175.40 ± 27.71 | 209.04 ± 14.87 |
| Insulin (ng/mL) | 1.43 ± 0.18 | 2.99 ± 0.67 * | 1.26 ± 0.22 | 4.08 ± 0.92 ** |
| FEMALES | ||||
| Supplemented Saline | Supplemented DHA | |||
| Standard Diet | fcHFHS Diet | Standard Diet | FcHFHS Diet | |
| Body weight (g) | 349 ± 80 | 451 ± 19 **** | 357 ± 11 | 407 ± 26 **** |
| Adipose Index | 7.33 ± 0.56 | 22.70 ± 0.42 **** | 7.67 ± 0.42 | 10.00 ± 1.59 *** |
| Leptin (ng/mL) | 6.53 ± 0.74 | 22.70 ± 3.04 *** | 6.81 ± 1.05 | 16.13 ± 3.10 * |
| Triglycerides (mg/dL) | 125.17 ± 13.27 | 339.99 ± 98.61 * | 140.59 ± 20.99 | 285.48 ± 36.99 * |
| Cholesterol (mg/dL) | 61.13 ± 3.91 | 85.11 ± 5.24 | 84.13 ± 4.10 §§ | 96.36 ± 3.83 |
| Glucose (mg/dL) | 171.65 ± 4.17 | 182.70 ± 10.07 | 183.89 ± 11.55 | 182.30 ± 13.42 |
| Insulin (ng/mL) | 0.93 ± 0.18 | 3.40 ± 0.76 ** | 1.45 ± 0.25 | 2.50 ± 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daher-Abdi, A.; Olvera Hernández, S.; Reyes Castro, L.A.; Mezo-González, C.E.; Croyal, M.; García-Santillán, J.A.; Ouguerram, K.; Zambrano, E.; Bolaños-Jiménez, F. Maternal DHA Supplementation during Pregnancy and Lactation in the Rat Protects the Offspring against High-Calorie Diet-Induced Hepatic Steatosis. Nutrients 2021, 13, 3075. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093075
Daher-Abdi A, Olvera Hernández S, Reyes Castro LA, Mezo-González CE, Croyal M, García-Santillán JA, Ouguerram K, Zambrano E, Bolaños-Jiménez F. Maternal DHA Supplementation during Pregnancy and Lactation in the Rat Protects the Offspring against High-Calorie Diet-Induced Hepatic Steatosis. Nutrients. 2021; 13(9):3075. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093075
Chicago/Turabian StyleDaher-Abdi, Amran, Sandra Olvera Hernández, Luis Antonio Reyes Castro, Carla Elena Mezo-González, Mikaël Croyal, Juan Antonio García-Santillán, Khadija Ouguerram, Elena Zambrano, and Francisco Bolaños-Jiménez. 2021. "Maternal DHA Supplementation during Pregnancy and Lactation in the Rat Protects the Offspring against High-Calorie Diet-Induced Hepatic Steatosis" Nutrients 13, no. 9: 3075. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093075






