α-Solanine Causes Cellular Dysfunction of Human Trophoblast Cells via Apoptosis and Autophagy
Abstract
:1. Introduction
2. Results
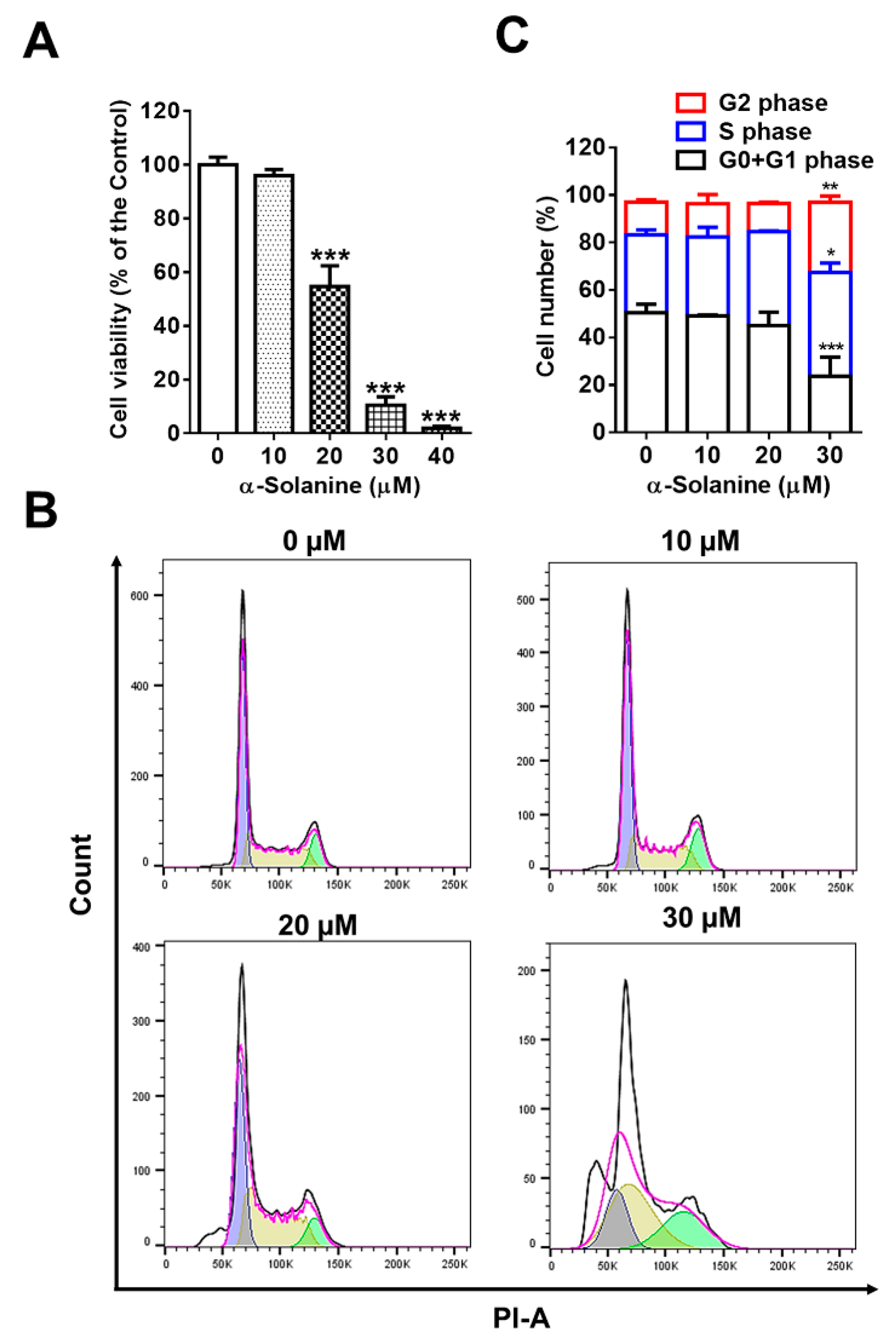
2.1. α-Solanine Inhibited Proliferation and Induced Cell Cycle Arrest of HTR-8/SVneo Cells
2.2. α-Solanine Induced Apoptosis in HTR-8/SVneo Cells
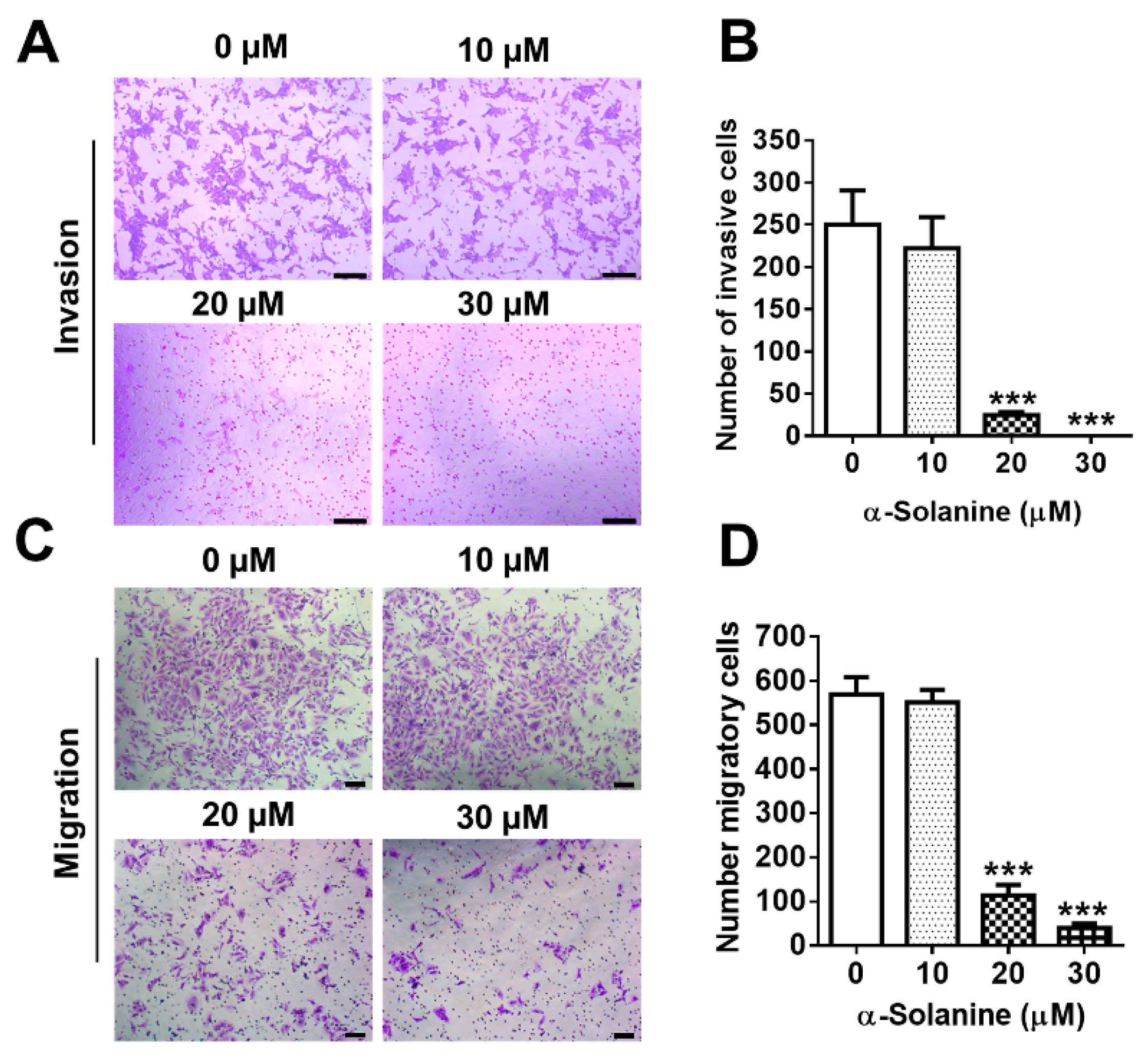
2.3. α-Solanine Inhibited Invasion and Migration of HTR-8/SVneo Cells
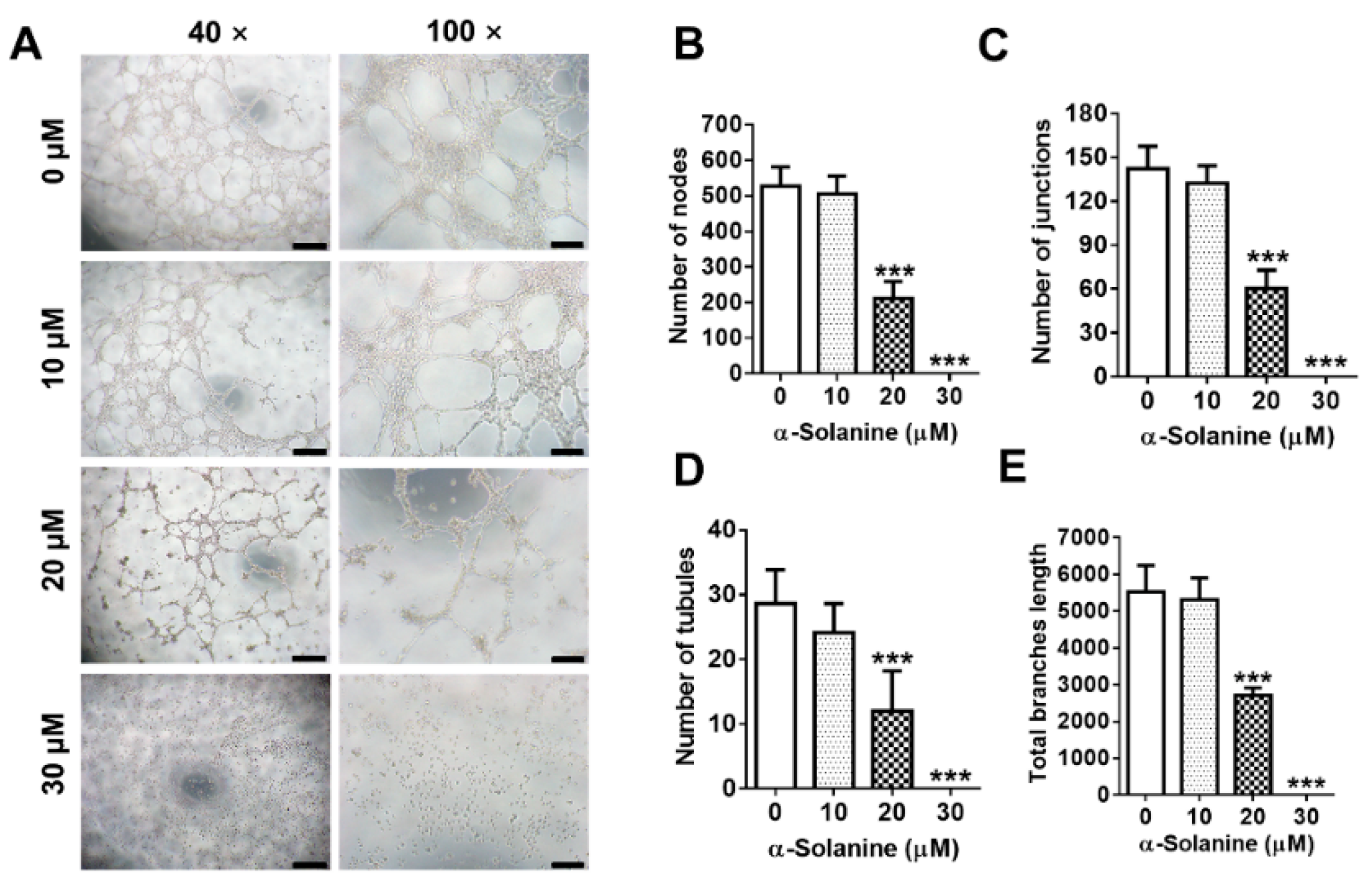
2.4. α-Solanine Inhibited the Endothelial-Like Tube Formation in HTR-8/SVneo Cells
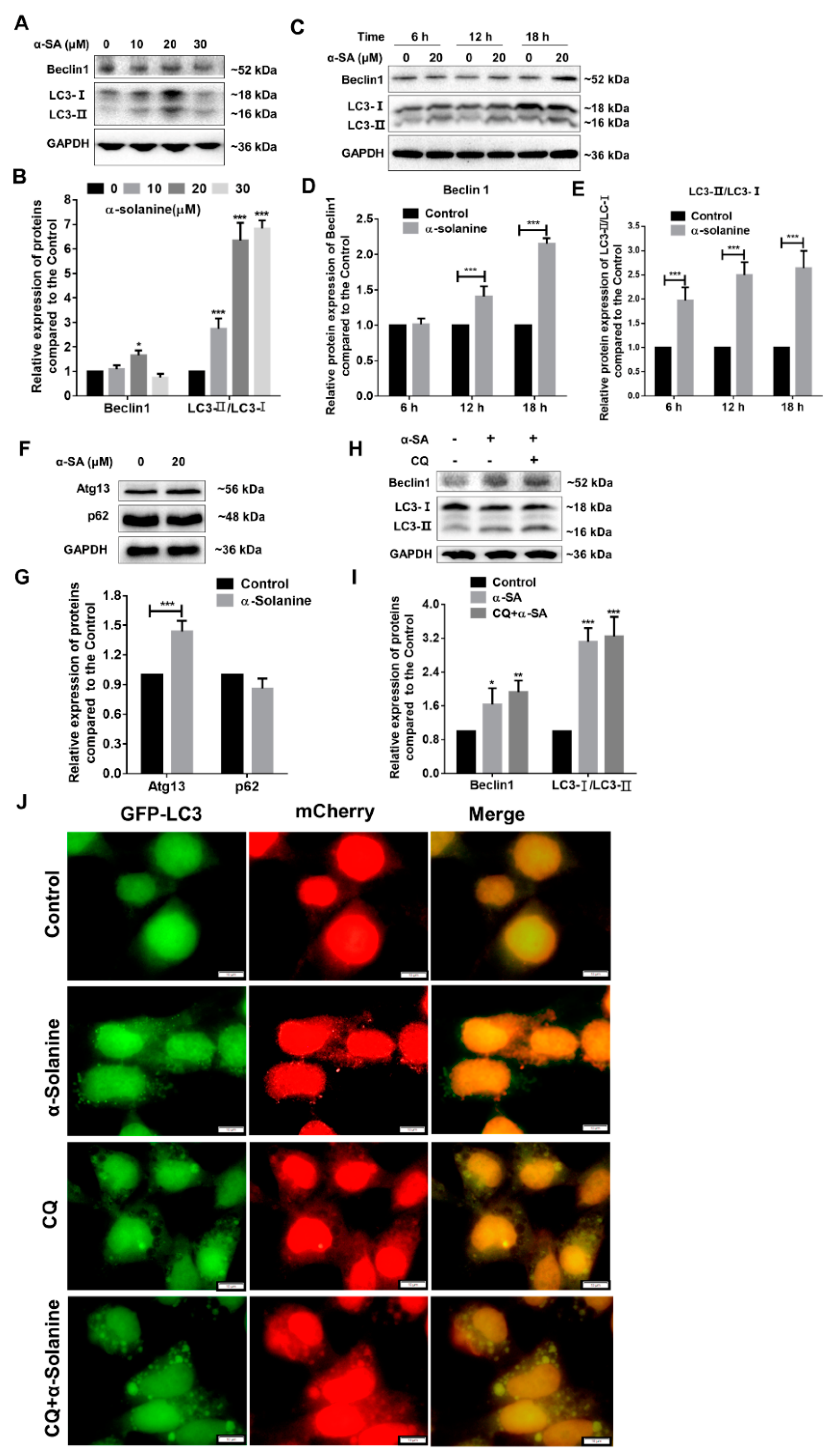
2.5. α-Solanine Triggered Autophagy in HTR-8/SVneo Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture
5.2. Cell Proliferation Assay
5.3. Cell Cycle Assay
5.4. Cell Invasion and Migration Assays
5.5. Tube Formation Assay
5.6. Cell Apoptosis Assay
5.7. TUNEL Assay
5.8. Western Blot
5.9. Autophagic Flux
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, R.; Zhang, G.; Cheng, R.; Bai, Y.; Zhao, H.; Lu, X.; Li, H.; Chen, S.; Li, J.; et al. Anticancer function of α-solanine in lung adenocarcinoma cells by inducing microRNA-138 expression. Tumor Biol. 2016, 37, 6437–6446. [Google Scholar] [CrossRef] [PubMed]
- Mohsenikia, M.; Farhangi, B.; Alizadeh, A.M.; Khodayari, H.; Khodayari, S.; Khori, V.; Arjmand Abbassi, Y.; Vesovic, M.; Soleymani, A.; Najafi, F. Therapeutic effects of dendrosomal solanine on a metastatic breast tumor. Life Sci. 2016, 148, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, L.; Du, X.; Sun, Q.; Wang, Y.; Li, M.; Zang, W.; Liu, K.; Zhao, G. α-solanine enhances the chemosensitivity of esophageal cancer cells by inducing microRNA-138 expression. Oncol. Rep. 2018, 39, 1163–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Sun, Q.Q.; Zhang, S.J.; Du, Y.W.; Wang, Y.Y.; Zang, W.Q.; Chen, X.N.; Zhao, G.Q. Inhibitory effect of α-solanine on esophageal carcinoma in vitro. Exp. Ther. Med. 2016, 12, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Kong, H.; Dong, G.; Liu, L.; Tong, K.; Sun, H.; Chen, B.; Zhang, C.; Zhou, M. Antitumor efficacy of α-solanine against pancreatic cancer in vitro and in vivo. PLoS ONE 2014, 9, e87868. [Google Scholar] [CrossRef]
- Shen, K.H.; Liao, A.C.; Hung, J.H.; Lee, W.J.; Hu, K.C.; Lin, P.T.; Liao, R.F.; Chen, P.S. α-Solanine inhibits invasion of human prostate cancer cell by suppressing epithelial-mesenchymal transition and MMPs expression. Molecules 2014, 19, 11896–11914. [Google Scholar] [CrossRef]
- Meng, X.Q.; Zha, W.; Zhang, F.; Yin, S.Y.; Xie, H.Y.; Zhou, L.; Zheng, S.S. Solanine-induced reactive oxygen species inhibit the growth of human hepatocellular carcinoma HepG2 cells. Oncol. Lett. 2016, 11, 2145–2151. [Google Scholar] [CrossRef] [Green Version]
- El-Daly, S.M.; Gouhar, S.A.; Gamal-Eldeen, A.M.; Abdel Hamid, F.F.; Ashour, M.N.; Hassan, N.S. Synergistic effect of α-Solanine and Cisplatin induces apoptosis and enhances cell cycle arrest in human hepatocellular carcinoma cells. Anticancer Agents Med. Chem. 2019, 19, 2197–2210. [Google Scholar] [CrossRef]
- Yi, Y.J.; Jia, X.H.; Wang, J.Y.; Chen, J.R.; Wang, H.; Li, Y.J. Solanine induced apoptosis and increased chemosensitivity to Adriamycin in T-cell acute lymphoblastic leukemia cells. Oncol. Lett. 2018, 15, 7383–7388. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.S.; Lee, K.G.; Lee, H.H.; Lee, H.J.; An, H.J.; Nam, J.H.; Jang, D.S.; Lee, K.T. α-Solanine isolated from Solanum Tuberosum L. cv Jayoung abrogates LPS-induced inflammatory responses via NF-κB inactivation in RAW 264.7 macrophages and endotoxin-induced shock model in mice. J. Cell Biochem. 2016, 117, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, L.; Di, S.N.; Xu, Q.; Ren, Q.C.; Chen, S.Z.; Huang, N.; Jia, D.; Shen, X.F. Steroidal alkaloid solanine A from Solanum Nigrum Linn. exhibits anti-inflammatory activity in lipopolysaccharide/interferon γ-activated murine macrophages and animal models of inflammation. Biomed. Pharmacother. 2018, 105, 606–615. [Google Scholar] [CrossRef]
- Friedman, M.; Mcdonald, G.M. Potato glycoalkaloids: Chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Langkilde, S.; Schrøder, M.; Stewart, D.; Meyer, O.; Conner, S.; Davies, H.; Poulsen, M. Acute toxicity of high doses of the glycoalkaloids, alpha-solanine and alpha-chaconine, in the Syrian Golden hamster. J. Agric. Food Chem. 2008, 56, 8753–8760. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, M.Y.; Song, G.; Lim, W. Alpha-solanine inhibits cell proliferation via mitochondrial dysfunction and inhibin synthesis in mouse testis in vitro and in vivo. Chemosphere 2019, 235, 271–279. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mackey, B.E. Effect of feeding solanidine, solasodine and tomatidine to non-pregnant and pregnant mice. Food Chem. Toxicol. 2003, 41, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Hellenäs, K.E.; Cekan, E.; Slanina, P.; Bergman, K. Studies of embryotoxicity and the incidence of external malformations after continuous intravenous infusion of alpha-chaconine in pregnant rats. Pharmacol. Toxicol. 1992, 70, 381–383. [Google Scholar] [CrossRef]
- Jelínek, R.; Kyzlink, V.; Blatiný, C., Jr. An evaluation of the embryotoxic effects of blighted potatoes on chicken embryos. Teratology 1976, 14, 335–342. [Google Scholar] [CrossRef]
- Wang, S.; Panter, K.E.; Gaffield, W.; Evans, R.C.; Bunch, T.D. Effects of steroidal glycoalkaloids from potatoes (Solanum tuberosum) on in vitro bovine embryo development. Anim. Reprod. Sci. 2005, 85, 243–250. [Google Scholar] [CrossRef]
- Lin, T.; Oqani, R.K.; Lee, J.E.; Kang, J.W.; Kim, S.Y.; Cho, E.S.; Jeong, Y.D.; Baek, J.J.; Jin, D.I. α-Solanine impairs oocyte maturation and quality by inducing autophagy and apoptosis and changing histone modifications in a pig model. Reprod. Toxicol. 2018, 75, 96–109. [Google Scholar] [CrossRef]
- Ji, L.; Brkić, J.; Liu, M.; Fu, G.; Peng, C.; Wang, Y.L. Placental trophoblast cell differentiation: Physiological regulation and pathological relevance to preeclampsia. Mol. Aspects Med. 2013, 34, 981–1023. [Google Scholar] [CrossRef] [PubMed]
- Correia-Branco, A.; Keating, E.; Martel, F. Placentation-related processes in a human first-trimester extravillous trophoblast cell line (HTR-8/SVneo cells) are affected by several xenobiotics. Drug Chem. Toxicol. 2019, 42, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Komine-Aizawa, S.; Hirohata, N.; Aizawa, S.; Abiko, Y.; Hayakawa, S. Porphyromonas gingivalis lipopolysaccharide inhibits trophoblast invasion in the presence of nicotine. Placenta 2015, 36, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.L.; Amaral, M.M.; Reppetti, J.; Damiano, A.E.; Ibarra, C.; Sacerdoti, F. Cytotoxic effects of Shiga toxin-2 on human extravillous trophoblast cell lines. Reproduction 2019, 157, 297–304. [Google Scholar] [CrossRef]
- Negi, M.; Mulla, M.J.; Han, C.S.; Abrahams, V.M. Allopurinol inhibits excess glucose-induced trophoblast IL-1β and ROS production. Reproduction 2020, 159, 73–80. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Spasic-Boskovic, O.; Jauniaux, E.; Charnock-Jones, D.S.; Burton, G.J. Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: Effects of antioxidant vitamins. Am. J. Pathol. 2007, 170, 1511–1520. [Google Scholar]
- Burdon, C.; Mann, C.; Cindrova-Davies, T.; Ferguson-Smith, A.C.; Burton, G.J. Oxidative stress and the induction of cyclooxygenase enzymes and apoptosis in the murine placenta. Placenta 2007, 28, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Huppertz, B.; Kadyrov, M.; Kingdom, J.C. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 2006, 195, 29–39. [Google Scholar] [CrossRef]
- Tomas, S.Z.; Prusac, I.K.; Roje, D.; Tadin, I. Trophoblast apoptosis in placentas from pregnancies complicated by preeclampsia. Gynecol. Obstet. Investig. 2011, 71, 250–255. [Google Scholar] [CrossRef]
- Maynard, S.E.; Karumanchi, S.A. Angiogenic factors and preeclampsia. Semin. Nephrol. 2011, 31, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Dokras, A.; Gardner, L.M.; Seftor, E.A.; Hendrix, M.J. Regulation of human cytotrophoblast morphogenesis by hepatocyte growth factor/scatter factor. Biol. Reprod. 2001, 65, 1278–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Yang, Q.; Zhang, H.; Lin, H.; Zhou, Z.; Lu, X.; Zhu, C.; Xue, L.Q.; Wang, H. Placenta specific protein 1 (PLAC1) is involved in the trophoblasts invasion and migration. Reproduction 2014, 148, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Carter, A.M.; Enders, A.C.; Pijnenborg, R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140070. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A.K. Leptin induces tube formation in first-trimester extravillous trophoblast cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 24–29. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef] [Green Version]
- Sucha, L.; Tomsik, P. The Steroidal Glycoalkaloids from Solanaceae: Toxic Effect, Antitumour Activity and Mechanism of Action. Planta Med. 2016, 82, 379–387. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, L.; Liang, Y.; Xiao, W.; Xie, L.; Zhang, Y.; Zhao, L.; Cao, L.; Chen, J.; Wang, G. Quantitative determination and pharmacokinetic study of solamargine in rat plasma by liquid chromatogramy-mass spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 1157–1162. [Google Scholar] [CrossRef]
- Blankemeyer, J.T.; McWilliams, M.L.; Rayburn, J.R.; Weissenberg, M.; Friedman, M. Developmental toxicology of solamargine and solasonine glycoalkaloids in frog embryos. Food Chem. Toxicol. 1998, 36, 383–389. [Google Scholar] [CrossRef]
- Friedman, M.; Fitch, T.E.; Yokoyama, W.E. Lowering of plasma LDL cholesterol in hamsters by the tomato glycoalkaloid tomatine. Food Chem. Toxicol. 2000, 38, 549–553. [Google Scholar] [CrossRef]
- Roddick, J.G. The steroidal glycoalkaloid α-tomatine. Phytochemistry 1974, 13, 9–25. [Google Scholar] [CrossRef]
- Nishie, K.; Norred, W.P.; Swain, A.P. Pharmacology and toxicology of chaconine and tomatine. Res. Commun. Chem. Pathol. Pharmacol. 1975, 12, 657–668. [Google Scholar]
- Mensinga, T.T.; Sips, A.J.; Rompelberg, C.J.; van Twillert, K.; Meulenbelt, J.; van den Top, H.J.; van Egmond, H.P. Potato glycoalkaloids and adverse effects in humans: An ascending dose study. Regul. Toxicol. Pharmacol. 2005, 41, 66–72. [Google Scholar] [CrossRef]
- Rayburn, J.R.; Friedman, M.; Bantle, J.A. Synergistic interaction of glycoalkaloids α-chaconine and α-solanine on developmental toxicity in Xenopus embryos. Food Chem. Toxicol. 1995, 33, 1013–1019. [Google Scholar] [CrossRef]
- Levy, R.; Nelson, D.M. To be, or not to be, that is the question. Apoptosis in human trophoblast. Placenta 2000, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Erboga, M.; Kanter, M. Effect of cadmium on trophoblast cell proliferation and apoptosis in different gestation periods of rat placenta. Biol. Trace Elem. Res. 2006, 169, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Terraneo, L.; Virgili, E.; Martinelli, C.; Doi, P.; Samaja, M.; Bulfamante, G.P.; Marconi, A.M. Autophagy in normal and abnormal early human pregnancies. Reprod. Sci. 2015, 22, 838–844. [Google Scholar] [CrossRef]
- Gao, L.; Qi, H.B.; Kamana, K.C.; Zhang, X.M.; Zhang, H.; Baker, P.N. Excessive autophagy induces the failure of trophoblast invasion and vasculature: Possible relevance to the pathogenesis of preeclampsia. J. Hypertens. 2015, 33, 106–117. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Shen, Y.C.; Kao, M.C.; Mathew, D.C.; Karuppaiya, P.; Li, M.L.; Yang, H.L. Ganoderma tsugae induced ROS-independent apoptosis and cytoprotective autophagy in human chronic myeloid leukemia cells. Food Chem. Toxicol. 2019, 124, 30–44. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Baehrecke, E.H.; Brumell, J.H.; Chu, C.T.; Codogno, P.; Cuervo, A.M.; Debnath, J.; Deretic, V.; Elazar, Z.; Eskelinen, E.L.; et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 2011, 7, 1273–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanain, M.; Bhattacharjee, A.; Pandey, P.; Ashraf, R.; Singh, N.; Sharma, S.; Vishwakarma, A.L.; Datta, D.; Mitra, K.; Sarkar, J. alpha-Solanine induces ROS-mediated autophagy through activation of endoplasmic reticulum stress and inhibition of Akt/mTOR pathway. Cell Death Dis. 2015, 6, e1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohue, E.; Balgi, A.D.; Komatsu, M.; Roberge, M. Induction of Covalently Crosslinked p62 Oligomers with Reduced Binding to Polyubiquitinated Proteins by the Autophagy Inhibitor Verteporfin. PLoS ONE 2014, 9, e114964. [Google Scholar] [CrossRef] [PubMed]
- B’chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Chen, S.P.; Zhang, X.P.; Wang, H.; Zhu, C.; Lin, H.Y. Smurf2 participates in human trophoblast cell invasion by inhibiting TGF-beta type I receptor. J. Histochem. Cytochem. 2009, 57, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Peng, X.; Yuan, A.; Xie, Y.; Yang, Q.; Xue, L. Peroxisome proliferator-activated receptor γ mediates porcine placental angiogenesis through hypoxia inducible factor-, vascular endothelial growth factor- and angiopoietin-mediated signaling. Mol. Med. Rep. 2017, 16, 2636–2644. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, J.; Yuan, A.; Han, J.; Tan, L.; Zhou, Z.; Zhao, H.; Su, R.; Huang, B.; Wang, B.; et al. R-spondin3 promotes the tumor growth of choriocarcinoma JEG-3 cells. Am. J. Physiol. Cell Physiol. 2020, 318, C664–C674. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, C.; Yuan, A.; Gu, T.; Zhang, F.; Fan, X.; Wu, X.; Xiong, X.; Yang, Q. α-Solanine Causes Cellular Dysfunction of Human Trophoblast Cells via Apoptosis and Autophagy. Toxins 2021, 13, 67. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13010067
Chen Z, Li C, Yuan A, Gu T, Zhang F, Fan X, Wu X, Xiong X, Yang Q. α-Solanine Causes Cellular Dysfunction of Human Trophoblast Cells via Apoptosis and Autophagy. Toxins. 2021; 13(1):67. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13010067
Chicago/Turabian StyleChen, Zhilong, Chen Li, Anwen Yuan, Ting Gu, Feng Zhang, Xiujun Fan, Xiaosong Wu, Xingyao Xiong, and Qing Yang. 2021. "α-Solanine Causes Cellular Dysfunction of Human Trophoblast Cells via Apoptosis and Autophagy" Toxins 13, no. 1: 67. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13010067




