The Effects of Zearalenone on the Localization and Expression of Reproductive Hormones in the Ovaries of Weaned Gilts
Abstract
:1. Introduction
2. Results
2.1. Vulva Size
2.2. Serum Hormones
2.3. Localizations of FSHR, LHR, GnRH and GnRHR
2.4. The mRNA and Protein Expressions
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals, Treatments and Feeding Management
5.2. Vulva Measurement
5.3. Serum and Ovary Samples Collection
5.4. Serum Hormone Measurement
5.5. Immunohistochemistry (IHC)
5.6. Integrated Optical Density Measurement
5.7. The mRNA Expression Using Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
5.8. Western Blotting
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautier, C.; Pinson-Gadais, L.; Richard-Forget, F. Fusarium Mycotoxins Enniatins: An updated review of their occurrence, the producing fusarium species, and the abiotic determinants of their accumulation in crop harvests. J. Agric. Food Chem. 2020, 68, 4788–4798. [Google Scholar] [CrossRef] [PubMed]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Jiao, R.; Peng, Z.; Li, Y.; Bai, W. Toxic effects of zearalenone on gametogenesis and embryonic development: A molecular point of review. Food Chem. Toxicol. 2018, 119, 24–30. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Ahmad, B.; Shrivastava, V.K.; Saleh, R.; Henkel, R.; Agarwal, A. Protective effects of saffron against zearalenone-induced alterations in reproductive hormones in female mice (Mus musculus). Clin. Exp. Reprod. Med. 2018, 45, 163–169. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, impact on agriculture, human health, and management strategies of zearalenone in food and feed: A review. Toxins (Basel) 2021, 13, 92. [Google Scholar] [CrossRef]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef] [Green Version]
- Jarrett, S.; Ashworth, C.J. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 2018, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Ropejko, K.; Twaruzek, M. Zearalenone and its metabolites-general overview, occurrence, and toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, L.; Chen, Y.; Sun, T.; Wang, N.; Chen, X.; Yang, Z.; Ge, J.; Jiang, S. Comparative study of stress response, growth and development of uteri in post-weaning gilts challenged with zearalenone and estradiol benzoate. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019, 103, 1885–1894. [Google Scholar] [CrossRef]
- Huang, W.; Yao, B.; Sun, L.; Pu, R.; Wang, L.; Zhang, R. Immunohistochemical and in situ hybridization studies of gonadotropin releasing hormone (GnRH) and its receptor in rat digestive tract. Life Sci. 2001, 68, 1727–1734. [Google Scholar] [CrossRef]
- Haen, S.M.; Heinonen, M.; Kauffold, J.; Heikinheimo, M.; Hoving, L.L.; Soede, N.M.; Peltoniemi, O.A.T. GnRH-agonist deslorelin implant alters the progesterone release pattern during early pregnancy in gilts. Reprod. Domest. Anim. 2019, 54, 464–472. [Google Scholar] [CrossRef]
- Kadokawa, H.; Pandey, K.; Nahar, A.; Nakamura, U.; Rudolf, F.O. Gonadotropin-releasing hormone (GnRH) receptors of cattle aggregate on the surface of gonadotrophs and are increased by elevated GnRH concentrations. Anim. Reprod. Sci. 2014, 150, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Yamamoto, T.; Matsubara, S.; Kawada, T.; Satake, H. Invertebrate gonadotropin-releasing hormone receptor signaling and its relevant biological actions. Int. J. Mol. Sci. 2020, 21, 8544. [Google Scholar] [CrossRef] [PubMed]
- Schang, A.L.; Querat, B.; Simon, V.; Garrel, G.; Bleux, C.; Counis, R.; Cohen-Tannoudji, J.; Laverriere, J.N. Mechanisms underlying the tissue-specific and regulated activity of the Gnrhr promoter in mammals. Front. Endocrinol. (Lausanne) 2012, 3, 162. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wu, W.; Pan, J.; Long, M. Detoxification strategies for zearalenone using microorganisms: A review. Microorganisms 2019, 7, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Gao, X.; Li, X.; Ye, Q.; Jebessa, E.; Abdalla, B.A.; Nie, Q. Molecular characterization, expression profile of the FSHR gene and its association with egg production traits in muscovy duck. J. Genet. 2017, 96, 341–351. [Google Scholar] [CrossRef]
- Menon, K.M.; Nair, A.K.; Wang, L.; Peegel, H. Regulation of luteinizing hormone receptor mRNA expression by a specific RNA binding protein in the ovary. Mol. Cell Endocrinol. 2007, 260-262, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Cui, Y.; Yu, S. Expression and localisation of FSHR, GHR and LHR in different tissues and reproductive organs of female yaks. Folia Morphol. (Warsz) 2018, 77, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Lan, R.X.; Liu, F.; He, Z.B.; Chen, C.; Liu, S.J.; Shi, Y.; Liu, Y.L.; Yoshimura, Y.; Zhang, M. Immunolocalization of GnRHRI, gonadotropin receptors, PGR, and PGRMCI during follicular development in the rabbit ovary. Theriogenology 2014, 81, 1139–1147. [Google Scholar] [CrossRef]
- Sengupta, A.; Chakrabarti, N.; Sridaran, R. Presence of immunoreactive gonadotropin releasing hormone (GnRH) and its receptor (GnRHR) in rat ovary during pregnancy. Mol. Reprod. Dev. 2008, 75, 1031–1044. [Google Scholar] [CrossRef]
- Wei, S.; Chen, S.; Gong, Z.; Ouyang, X.; An, L.; Xie, K.; Dong, J.; Wei, M. Alarelin active immunization influences expression levels of GnRHR, FSHR and LHR proteins in the ovary and enhances follicular development in ewes. Anim. Sci. J. 2013, 84, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xie, J.; Zhou, L.; Fan, Y.; Yu, B.; Chen, Q.; Fu, Y.; Yan, Z.; Guo, H.; Lyu, Q.; et al. The role of combining medroxyprogesterone 17-acetate with human menopausal gonadotropin in mouse ovarian follicular development. Sci. Rep. 2018, 8, 4439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Eppley, R.M.; Alam, H.Z.; Rorie, J.; Ruggles, D.I. Effects of zearalenone on in utero development in rats. Food Chem. Toxicol. 2006, 44, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Y.M.; Zhang, L.; Zhao, Z.M.; Zhao, J.; Peng, S.Q. Prepubertal exposure to an oestrogenic mycotoxin zearalenone induces central precocious puberty in immature female rats through the mechanism of premature activation of hypothalamic kisspeptin-GPR54 signaling. Mol. Cell Endocrinol. 2016, 437, 62–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Yin, S.; Shan, A.; Gao, R.; Qu, Z.; Liu, M.; Nie, S. Toxic effects of maternal zearalenone exposure on uterine capacity and fetal development in gestation rats. Reprod. Sci. 2014, 21, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Gajecka, M.; Rybarczyk, L.; Jakimiuk, E.; Zielonka, L.; Obremski, K.; Zwierzchowski, W.; Gajecki, M. The effect of experimental long-term exposure to low-dose zearalenone on uterine histology in sexually immature gilts. Exp. Toxicol. Pathol. 2012, 64, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, M.Y.; Zhao, A.H.; Kong, L.; Wang, J.J.; Shen, W.; Li, L. Single-cell transcriptomic profiling provides insights into the toxic effects of zearalenone exposure on primordial follicle assembly. Theranostics 2021, 11, 5197–5213. [Google Scholar] [CrossRef]
- Wang, J.P.; Chi, F.; Kim, I.H. Effects of montmorillonite clay on growth performance, nutrient digestibility, vulva size, faecal microflora, and oxidative stress in weaning gilts challenged with zearalenone. Anim. Feed Sci. Technol. 2012, 178, 158–166. [Google Scholar] [CrossRef]
- Fu, G.; Ma, J.; Wang, L.; Yang, X.; Liu, J.; Zhao, X. Effect of degradation of zearalenone-contaminated feed by Bacillus licheniformis CK1 on postweaning female oiglets. Toxins 2016, 8, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Sun, Y.; Ju, D.; Chang, S.; Shi, B.; Shan, A. The detoxification effect of vitamin C on zearalenone toxicity in piglets. Ecotoxicol. Environ. Saf. 2018, 158, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Gao, J.; Liu, F.X.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011, 89, 3008–3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farage, M.; Maibach, H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 2006, 273, 195–202. [Google Scholar] [CrossRef]
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ. Int. 2015, 83, 11–40. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.X.; Bu, Y.; Bottum, K.M.; Tischkau, S.A. Beta-naphthoflavone (DB06732) mediates estrogen receptor-positive breast cancer cell cycle arrest through AhR-dependent regulation of PI3K/AKT and MAPK/ERK signaling. Carcinogenesis 2014, 35, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oguz, H.; Kececi, T.; Birdane, Y.O.; Onder, F.; Kurtoglu, V. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. Res. Vet. Sci. 2000, 69, 89–93. [Google Scholar] [CrossRef]
- Gao, X.; Sun, L.; Zhang, N.; Li, C.; Zhang, J.; Xiao, Z.; Qi, D. Gestational zearalenone exposure causes reproductive and developmental toxicity in pregnant rats and female offspring. Toxins 2017, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Moenter, S.M.; Karsch, F.J.; Lehman, M.N. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: Induction in GnRH and other neurons. Endocrinology 1993, 133, 896–903. [Google Scholar] [CrossRef]
- Shi, D.; Zhou, J.; Zhao, L.; Rong, X.; Fan, Y.; Hamid, H.; Li, W.; Ji, C.; Ma, Q. Alleviation of mycotoxin biodegradation agent on zearalenone and deoxynivalenol toxicosis in immature gilts. J. Anim. Sci. Biotechnol. 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, N.P.; Dahl, G.E.; Glover, B.H.; Karsch, F.J. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 1994, 134, 1806–1811. [Google Scholar] [CrossRef]
- Lai, L.; Shen, X.; Liang, H.; Deng, Y.; Gong, Z.; Wei, S. Determine the role of FSH receptor binding inhibitor in regulating ovarian follicles development and expression of FSHR and ERalpha in mice. BioMed Res. Int. 2018, 2018, 5032875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milazzotto, M.P.; Rahal, P.; Nichi, M.; Miranda-Neto, T.; Teixeira, L.A.; Ferraz, J.B.S.; Eler, J.P.; Campagnari, F.; Garcia, J.F. New molecular variants of hypothalamus–pituitary–gonad axis genes and their association with early puberty phenotype in Bos taurus indicus (Nellore). Livest. Sci. 2008, 114, 274–279. [Google Scholar] [CrossRef]
- Zhang, Q.; Madden, N.E.; Wong, A.S.T.; Chow, B.K.C.; Lee, L.T.O. The role of endocrine G protein-coupled receptors in ovarian cancer progression. Front. Endocrinol. (Lausanne) 2017, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Suocheng, W.; Zhuandi, G.; Li, S.; Haoqin, L.; Luju, L.; Yingying, D. Maturation rates of oocytes and levels of FSHR, LHR and GnRHR of COCs response to FSH concentrations in IVM media for sheep. J. Appl. Biomed. 2017, 15, 180–186. [Google Scholar] [CrossRef]
- Silva, J.R.; van den Hurk, R.; de Matos, M.H.; dos Santos, R.R.; Pessoa, C.; de Moraes, M.O.; de Figueiredo, J.R. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology 2004, 61, 1691–1704. [Google Scholar] [CrossRef]
- Kriszt, R.; Winkler, Z.; Polyak, A.; Kuti, D.; Molnar, C.; Hrabovszky, E.; Kallo, I.; Szoke, Z.; Ferenczi, S.; Kovacs, K.J. Xenoestrogens ethinyl estradiol and zearalenone cause precocious puberty in female rats via central isspeptin signaling. Endocrinology 2015, 156, 3996–4007. [Google Scholar] [CrossRef] [Green Version]
- Knox, R.V.; Webel, S.K.; Swanson, M.; Johnston, M.E.; Kraeling, R.R. Effects of estrus synchronization using Matrix® followed by treatment with the GnRH agonist triptorelin to control ovulation in mature gilts. Anim. Reprod. Sci. 2017, 185, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xiao, Y.; Ye, C.; Jia, J.; Liu, X.; Liang, H.; Zou, G.; Hu, G. Pituitary Action of E2 in Prepubertal grass carp: Receptor specificity and signal transduction for luteinizing hormone and follicle-stimulating hormone regulation. Front. Endocrinol. (Lausanne) 2018, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Terasawa, E. Neuroestradiol in regulation of GnRH release. Horm. Behav. 2018, 104, 138–145. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Yang, Z.; Yang, W.; Huang, L.; Wang, S.; Liu, F.; Liu, M.; Wang, Y.; Jiang, S. Effects of dietary zearalenone exposure on the growth performance, small intestine disaccharidase, and antioxidant activities of weaned gilts. Animals 2020, 10, 2157. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Liu, X.; Yuan, X.; Yang, W.; Liu, F.; Hou, Y.; Huang, L.; Jiang, S. Dose-effect of zearalenone on the localization and expression of growth hormone, growth hormone receptor, and heat shock protein 70 in the ovaries of post-weaning gilts. Front. Vet. Sci. 2021, 8, 629006. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Yang, C.W.; Huang, L.B.; Niu, Q.S.; Jiang, S.Z.; Chi, F. Zearalenone altered the serum hormones, morphologic and apoptotic measurements of genital organs in post-weaning gilts. Asian-Australas J. Anim. Sci. 2015, 28, 171–179. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- AOAC. Official Methods of Analysis of the AOAC; AOAC International: Rockville, MD, USA, 2012. [Google Scholar]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Liu, F.X.; Johnston, L.A.; Chi, F.; Wang, Y. Effect of purified zearalenone with or without modified montmorillonite on nutrient availability, genital organs and serum hormones in post-weaning piglets. Livest. Sci. 2012, 144, 110–118. [Google Scholar] [CrossRef]
- Rivera, A.; Agnati, L.F.; Horvath, T.L.; Valderrama, J.J.; Calle, A.D.L.; Fuxe, K. Uncoupling protein 2/3 immunoreactivity and the ascending dopaminergic and noradrenergic neuronal systems: Relevance for volume transmission. Neuroscience 2006, 137, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, L.; Shao, M.; Wang, Y.; Yang, W.; Huang, L.; Zhou, X.; Jiang, S.; Yang, Z. Effects of zearalenone exposure on the TGF-beta1/Smad3 signaling pathway and the expression of proliferation or apoptosis related genes of post-weaning gilts. Toxins 2018, 10, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
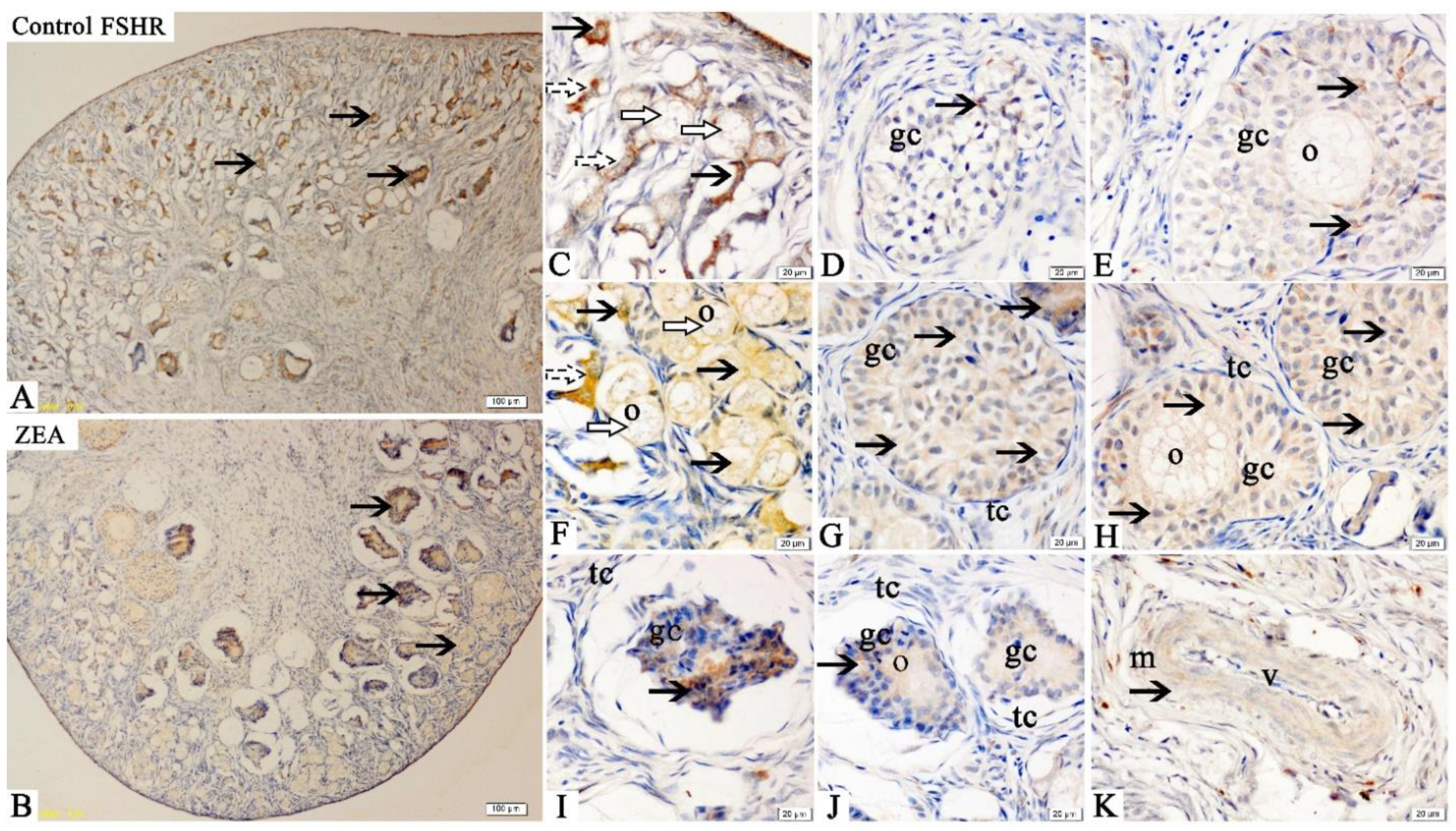
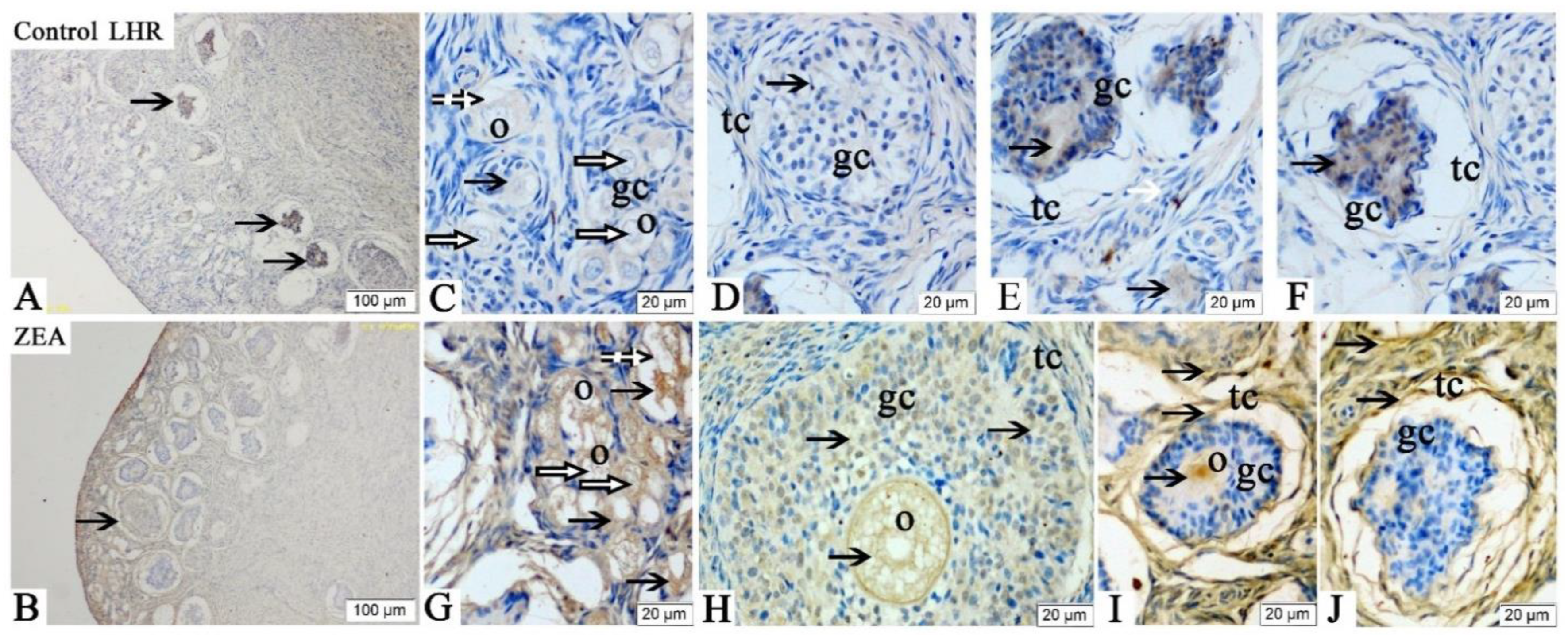
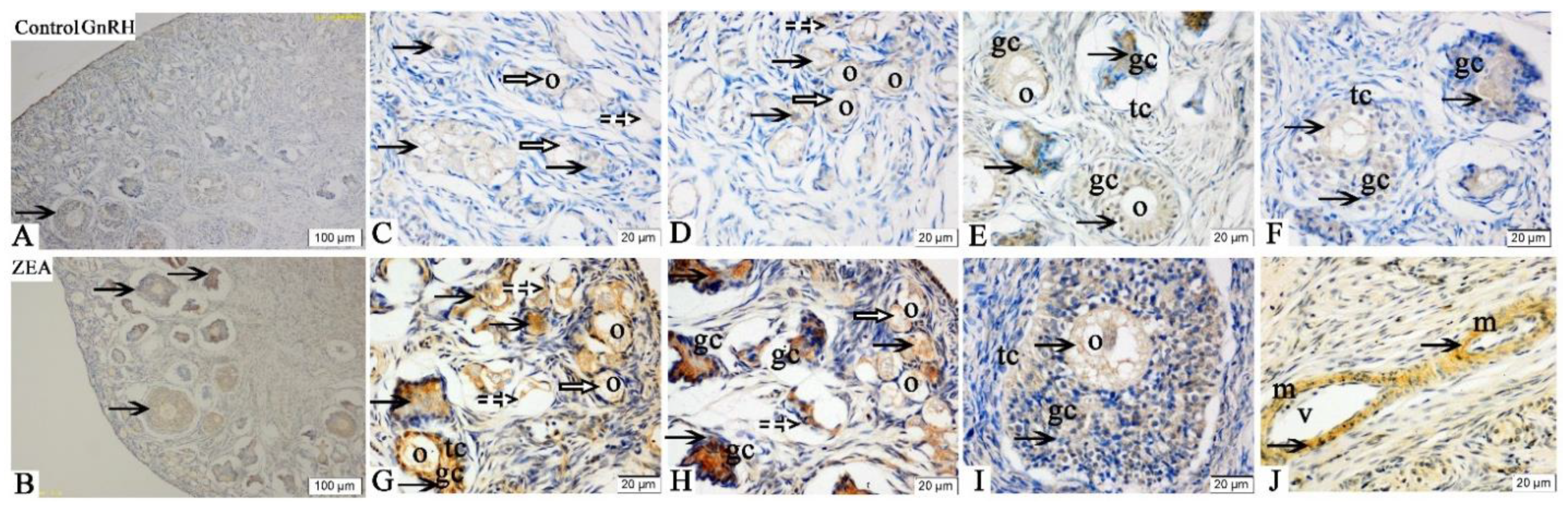
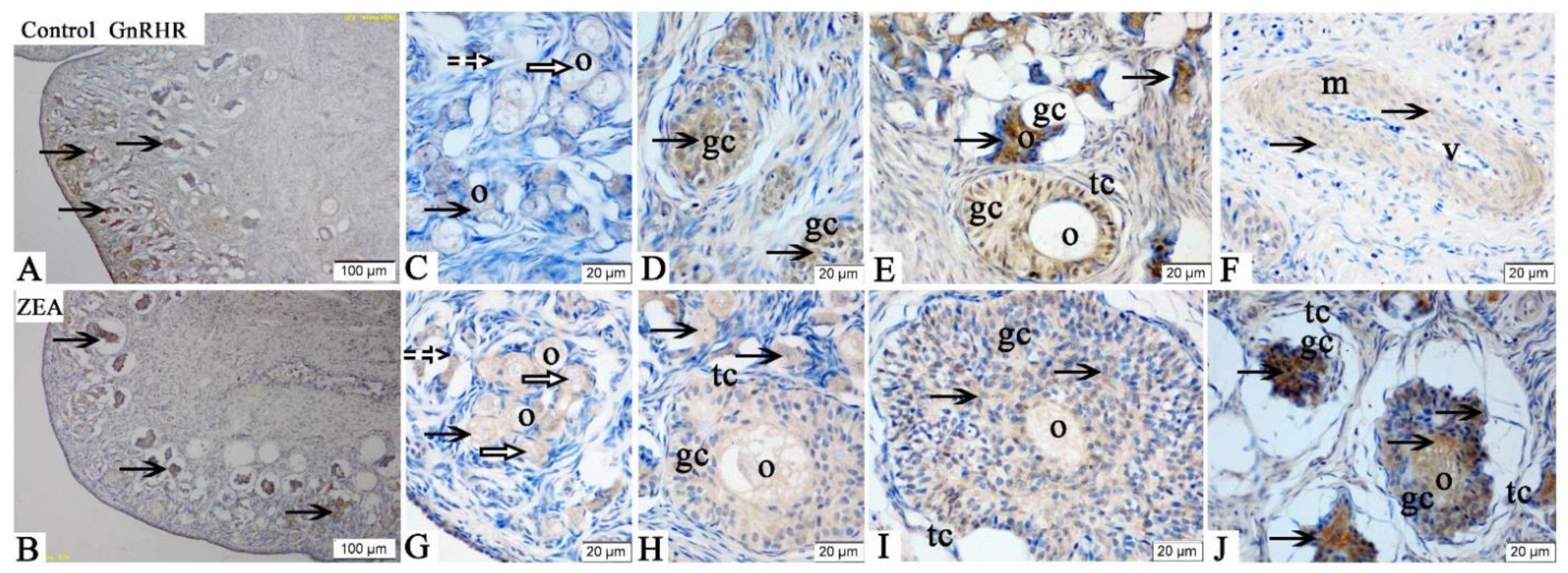

| Items | Control | ZEA |
|---|---|---|
| Vulva size, cm2 | ||
| Initial area | 1.39 ± 0.14 | 1.27 ± 0.12 |
| Final area | 2.78 ± 0.09 b | 4.89 ± 0.18 a |
| Final weight, kg | 29.38 ± 2.60 | 28.66 ± 2.55 |
| Final area/initial area | 2.03 ± 0.25 b | 3.89 ± 0.32 a |
| Final area/final weight, cm2/kg | 0.10 ± 0.01 b | 0.17 ± 0.02 a |
| Serum hormones | ||
| Estradiol, ng/mL | 11.19 ± 0.11 b | 14.27 ± 0.10 a |
| Progesterone, ng/mL | 1.23 ± 0.08 | 1.19 ± 0.06 |
| Follicle stimulating hormone, mIU/mL | 2. 87 ± 0.09 b | 4.19 ± 0.18 a |
| Luteinizing hormone, mIU/mL | 3.39 ± 0.11 | 3.62 ± 0.21 |
| Gonadotropin releasing hormone, ng/L | 12.29 ± 0.27 b | 17.52 ± 0.48 a |
| Items | IOD ×103 (n = 10) | mRNA Expression (n = 6) | ||
|---|---|---|---|---|
| Control | ZEA | Control | ZEA | |
| Estradiol | 5.28 ± 0.36 b | 11.33 ± 1.53 a | 1.00 ± 0.12 b | 3.19 ± 0.12 a |
| Progesterone | 4.32 ± 0.35 b | 4.72 ± 0.16 a | 1.00 ± 0.09 b | 1.53 ± 0.04 a |
| Follicle stimulating hormone | 2.11 ± 0.26 b | 4.81 ± 0.34 a | 1.00 ± 0.07 b | 2.69 ± 0.21 a |
| Luteinizing hormone | 2.89 ± 0.15 b | 3.13 ± 0.21 a | 1.00 ± 0.13 b | 1.96 ± 0.17 a |
| Ingredients | Content (%) | Nutrients | Analyzed Values (%) |
|---|---|---|---|
| Corn | 53.00 | Metabolizable energy, MJ/kg | 13.22 |
| Wheat middling | 5.00 | Crude protein | 19.40 |
| Whey powder | 6.50 | Calcium | 0.84 |
| Soybean oil | 2.50 | Total phosphorus | 0.73 |
| Soybean meal | 24.76 | Lysine | 1.36 |
| Fish meal | 5.50 | Methionine | 0.46 |
| L-Lysine HCl | 0.30 | Sulfur amino acid | 0.79 |
| DL-methionine | 0.10 | Threonine | 0.90 |
| L-threonine | 0.04 | Tryptophan | 0.25 |
| Calcium phosphate | 0.80 | ||
| Limestone, Pulverized | 0.30 | ||
| Sodium chloride | 0.20 | ||
| Premix 1 | 1.00 | ||
| Total | 100 |
| Target Genes | Primer Sequence (5′ to 3′) | Product Size | Accession No. |
|---|---|---|---|
| GADPH | F: ATGGTGAAGGTCGGAGTGAA | 154 | NM_001206359.1 |
| R: CGTGGGTGGAATCATACTGG | |||
| FSHR | F: ATGTCCTTGCTCCTGGTGTC | 213 | NM_214386.1 |
| R: GGTCCCCAAATCCAGAAAAT | |||
| LHR | F: GAAAGCACAGCAAGGAGACC | 282 | NM_214449.1 |
| R: ACATGAGGAAACGAGGCACT | |||
| GnRH | F: AGCCAACACTGGTCCTATCGATTG | 206 | NM_214274.1 |
| R: GTCTTCTGCCCAGTTTCCTCTTCA | |||
| GnRHR | F: AGCCAACCTGTTGGAGACTCTGAT | 101 | NM_214273 |
| R: AGCTGAGGACTTTGCAGAGGAACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, B.; Yuan, X.; Yang, W.; Jiao, N.; Li, Y.; Liu, F.; Liu, M.; Yang, Z.; Huang, L.; Jiang, S. The Effects of Zearalenone on the Localization and Expression of Reproductive Hormones in the Ovaries of Weaned Gilts. Toxins 2021, 13, 626. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090626
Wan B, Yuan X, Yang W, Jiao N, Li Y, Liu F, Liu M, Yang Z, Huang L, Jiang S. The Effects of Zearalenone on the Localization and Expression of Reproductive Hormones in the Ovaries of Weaned Gilts. Toxins. 2021; 13(9):626. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090626
Chicago/Turabian StyleWan, Boyang, Xuejun Yuan, Weiren Yang, Ning Jiao, Yang Li, Faxiao Liu, Mei Liu, Zaibin Yang, Libo Huang, and Shuzhen Jiang. 2021. "The Effects of Zearalenone on the Localization and Expression of Reproductive Hormones in the Ovaries of Weaned Gilts" Toxins 13, no. 9: 626. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13090626





