Autologous Transplantation for Older Adults with AML
Abstract
:1. Introduction
2. Is Autologous Hematopoietic Cell Transplantation Safe in Older AML Patients?
3. Are Results after Autologous Hematopoietic Cell Transplantation in Older AML Patients Comparable to Younger Patients?
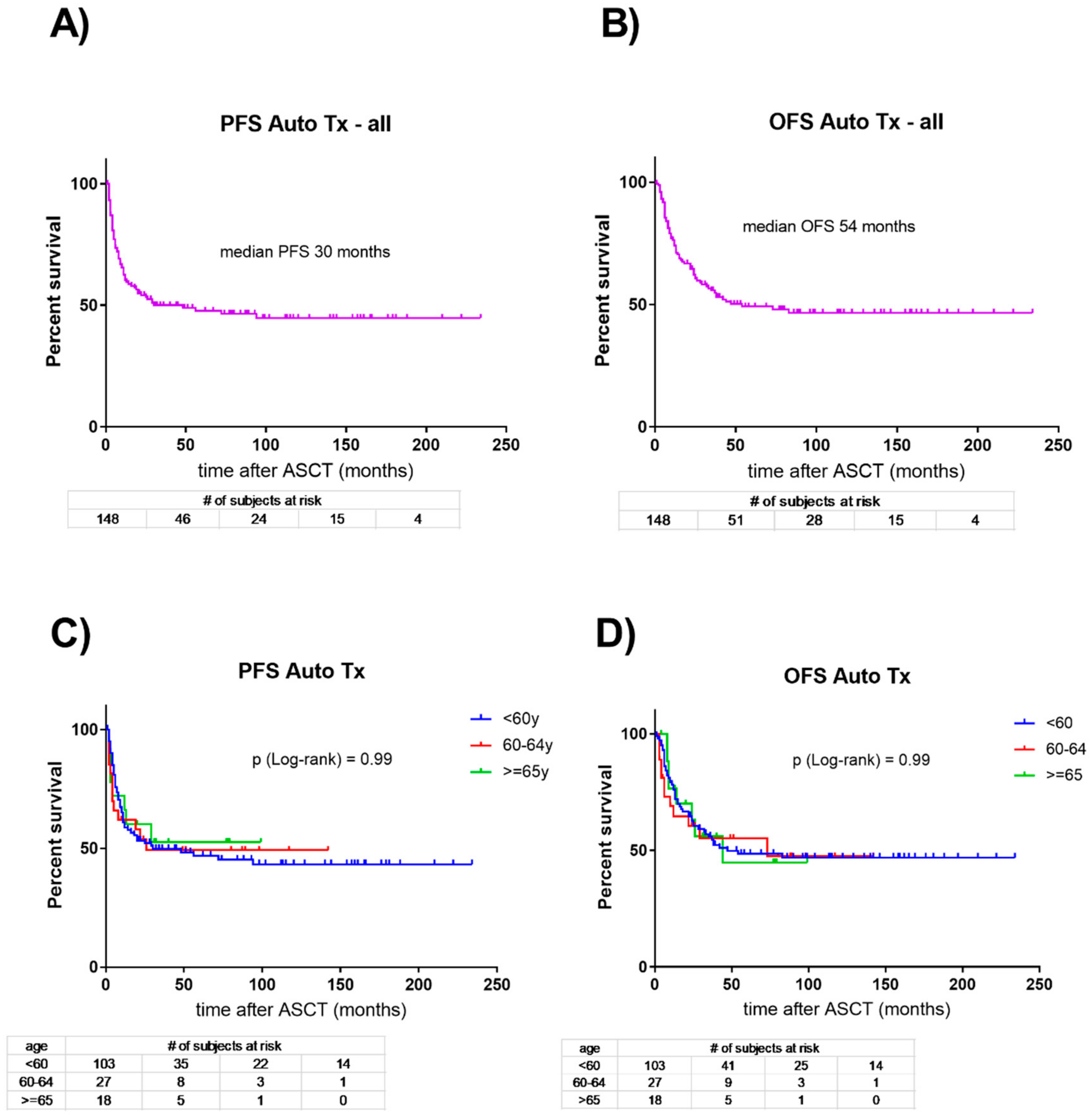
4. How Does Consolidation with Autologous Hematopoietic Cell Transplantation Compare to No Consolidation in Older AML Patients?
5. Is Consolidation of First Remission with Autologous Hematopoietic Cell Transplantation Superior to Intensive Chemotherapy in Older AML Patients?
6. Is Consolidation of First Remission with Autologous Hematopoietic Cell Transplantation Inferior to Allogeneic Transplantation in Older AML Patients?
7. Does Molecular and Cytogenetic Risk Stratification Change the above Conclusions?
8. Are Autologous Stem Cells more Difficult to Collect in Older AML Patients?
9. How to Proceed with Older AML Patients Failing Peripheral Stem Cell Mobilization?
10. Is There a Preferred Conditioning Regimen for Autologous Hematopoietic Cell Transplantation in Older AML Patients?
11. Concluding Remarks: Is Autologous Hematopoietic Cell Transplantation an Option for Older Patients with AML?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dohner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, G.; Lowenberg, B. How I treat the older patient with acute myeloid leukemia. Blood 2015, 125, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Lowenberg, B.; Ossenkoppele, G.J.; van Putten, W.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Maertens, J.; Jongen-Lavrencic, M.; von Lilienfeld-Toal, M.; et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Vellenga, E.; van Putten, W.; Ossenkoppele, G.J.; Verdonck, L.F.; Theobald, M.; Cornelissen, J.J.; Huijgens, P.C.; Maertens, J.; Gratwohl, A.; Schaafsma, R.; et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood 2011, 118, 6037–6042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelissen, J.J.; Blaise, D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood 2016, 127, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, D.; Mueller, B.U.; Mansouri Taleghani, B.; Baerlocher, G.M.; Seipel, K.; Leibundgut, K.; Pabst, T. Delayed Haematological recovery after autologous stem cell transplantation is associated with favourable outcome in acute myeloid leukaemia. Br. J. Haematol. 2015, 168, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Cahn, J.Y.; Labopin, M.; Mandelli, F.; Goldstone, A.H.; Eberhardt, K.; Reiffers, J.; Ferrant, A.; Franklin, I.; Hervé, P.; Gratwohl, A. Autologous bone marrow transplantation for first remission acute myeloblastic leukemia in patients older than 50 years: A retrospective analysis of the European Bone Marrow Transplant Group. Blood 1995, 85, 575–579. [Google Scholar] [PubMed]
- Gorin, N.C.; Aegerter, P.; Auvert, B.; Meloni, G.; Goldstone, A.H.; Burnett, A.; Carella, A.; Korbling, M.; Herve, P.; Maraninchi, D. Autologous bone marrow transplantation for acute myelocytic leukemia in first remission: A European survey of the role of marrow purging. Blood 1990, 75, 1606–1614. [Google Scholar] [PubMed]
- Herr, A.L.; Labopin, M.; Blaise, D.; Milpied, N.; Potter, M.; Michallet, M.; Heit, W.; Ferrara, F.; Esteve, J.; Arcese, W.; et al. HLA-identical sibling allogeneic peripheral blood stem cell transplantation with reduced intensity conditioning compared to autologous peripheral blood stem cell transplantation for elderly patients with de novo acute myeloid leukemia. Leukemia 2007, 21, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Oriol, A.; Ribera, J.M.; Esteve, J.; Guàrdia, R.; Brunet, S.; Bueno, J.; Pedro, C.; Llorente, A.; Tormo, M.; Besalduch, J.; et al. Feasibility and results of autologous stem cell transplantation in de novo acute myeloid leukemia in patients over 60 years old. Results of the CETLAM AML-99 protocol. Haematologica 2004, 89, 791–800. [Google Scholar] [PubMed]
- Heini, A.D.; Berger, M.D.; Seipel, K.; Taleghani, B.M.; Baerlocher, G.M.; Leibundgut, K.; Banz, Y.; Novak, U.; Pabst, T. Consolidation with autologous stem cell transplantation in first remission is safe and effective in AML patients above 65 years. Leuk. Res. 2017, 53, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelissen, J.J.; Versluis, J.; Passweg, J.R.; van Putten, W.L.; Manz, M.G.; Maertens, J.; Beverloo, H.B.; Valk, P.J.; van Marwijk Kooy, M.; Wijermans, P.W.; et al. Comparative therapeutic value of postremission approaches in patients with acute myeloid leukemia aged 40–60 years. Leukemia 2015, 29, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Stelljes, M.; Krug, U.; Beelen, D.W.; Braess, J.; Sauerland, M.C.; Heinecke, A. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: A prospective matched pairs analysis. J. Clin. Oncol. 2014, 32, 288–296. [Google Scholar] [CrossRef] [PubMed]
- McClune, B.L.; Weisdorf, D.J.; Pedersen, T.L.; Tunes da Silva, G.; Tallman, M.S.; Sierra, J. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J. Clin. Oncol. 2010, 28, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Sandmaier, B.M.; Storer, B.E.; Franke, G.N.; Laport, G.G.; Chauncey, T.R. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA 2011, 306, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Saraceni, F.; Labopin, M.; Gorin, N.C. Matched and mismatched unrelated donor compared to autologous stem cell transplantation for acute myeloid leukemia in first complete remission: A retrospective, propensity score weighted analysis from the ALWP of the EBMT. J. Hematol. Oncol. 2016, 9, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Hara, M.; Fujita, H. Comparable outcomes between autologous and allogeneic transplant for adult acute myeloid leukemia in first CR. Bone Marrow Transplant. 2016, 51, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorin, N.C.; Labopin, M.; Piemontese, S.; Arcese, W.; Santarone, S.; Huang, H.; Meloni, G.; Ferrara, F.; Beelen, D.; Sanz, M.; et al. Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. T-cell-replete haploidentical transplantation versus autologous stem cell transplantation in adult acute leukemia: A matched pair analysis. Haematologica 2015, 100, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Versluis, J.; In ’t Hout, F.E.; Devillier, R.; van Putten, W.L.; Manz, M.G.; Vekemans, M.C.; Legdeur, M.C.; Passweg, J.R.; Maertens, J.; Kuball, J.; et al. Comparative value of postremission treatment in cytogenetically normal AML subclassified by NPM1 and FLT3-ITD allelic ratio. Leukemia 2017, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Saraceni, F.; Bruno, B.; Lemoli, R.M.; Meloni, G.; Arcese, W.; Falda, M.; Ciceri, F.; Alessandrino, E.P.; Specchia, G.; Scimè, R.; et al. Autologous stem cell transplantation is still a valid option in good- and intermediate-risk AML: AGITMO survey on 809 patients autografted in first complete remission. Bone Marrow Transplant. 2017, 52, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Viola, A.; Copia, C.; Falco, C.; D’Elia, R.; Tambaro, F.P.; Correale, P.; D’Amico, M.R.; Vicari, L.; Palmieri, S. Age has no influence on mobilization of peripheral blood stem cells in acute myeloid leukemia. Hematol. Oncol. 2007, 25, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, M.H.; Kim, S.A.; Chang, J.S. Age-related deterioration of hematopoietic stem cells. Int. J. Stem Cells 2008, 1, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hengeveld, M.; Suciu, S.; Chelgoum, Y.; Marie, J.P.; Muus, P.; Lefrère, F. High numbers of mobilized CD34+ cells collected in AML in first remission are associated with high relapse risk irrespective of treatment with autologous peripheral blood SCT or autologous BMT. Bone Marrow Transplant. 2015, 50, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Visani, G.; Lemoli, R.; Tosi, P.; Martinelli, G.; Testoni, N.; Ricci, P. Use of peripheral blood stem cells for autologous transplantation in acute myeloid leukemia patients allows faster engraftment and equivalent disease-free survival compared with bone marrow cells. Bone Marrow Transplant. 1999, 24, 467–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, N.; Damon, L.; Varosy, P.; Navarro, W.; Martin, T.; Ries, C.; Linker, C. High CD34+ cell dose promotes faster platelet recovery after autologous stem cell transplantation for acute myeloid leukemia. Biol. Blood Marrow Transplant. 2003, 9, 643–648. [Google Scholar] [CrossRef]
- Von Grunigen, I.; Raschle, J.; Rusges-Wolter, I.; Mansouri Taleghani, B.M.; Mueller, B.U.; Pabst, T. The relapse risk of AML patients undergoing autlogous transplantation correlates with the stem cell mobilizing potential. Leuk. Res. 2012, 36, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.; Suciu, S.; de Witte, T.; Zittoun, R.; Mandelli, F.; Belhabri, A.; Amadori, S.; Fibbe, W.; Gallo, E.; Fillet, G.; et al. The stem cell mobilizing capacity of patients with acute myeloid leukemia in complete remission correlates with relapse risk: Results of the EORTC-GIMEMA AML-10 trial. Leukemia 2003, 17, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Raschle, J.; Ratschiller, D.; Mans, S.; Mueller, B.U.; Pabst, T. High levels of circulating CD34+ cells at autologous stem cell collection are associated with favourable prognosis in multiple myeloma. Br. J. Cancer 2011, 105, 970–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorin, N.C.; Labopin, M.; Blaise, D.; Reiffers, J.; Meloni, G.; Michallet, M.; de Witte, T.; Attal, M.; Rio, B.; Witz, F.; et al. Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. Higher incidence of relapse with peripheral blood rather than marrow as a source of stem cells in adults with acute myelocytic leukemia autografted during the first remission. J. Clin. Oncol. 2009, 27, 3987–3993. [Google Scholar] [PubMed]
- Feller, N.; Schuurhuis, G.J.; van der Pol, M.A.; Westra, G.; Weijers, G.W.; van Stijn, A.; Huijgens, P.C.; Ossenkoppele, G.J. High percentage of CD34-positive cells in autologous AML peripheral blood stem products reflects inadequate in vivo purging and low chemotherapeutic toxicity in a subgroup of patients with poor clinical outcome. Leukemia 2003, 17, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Blum, V.; Heini, A.D.; Novak, U.; Taleghani, B.M.; Baerlocher, G.M.; Leibundgut, K.; Seipel, K.; Banz, Y.; Bargetzi, M.; Pabst, T. Hematopoietic stem cell remobilization with vinorelbine and filgrastim in AML. Bone Marrow Transplant. 2017, 52, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Bik To, L.; Levesque, J.P.; Herbert, K.E. How I treat patients who mobilize hematopoietic stem cells poorly. Blood 2011, 118, 4530–4540. [Google Scholar] [PubMed] [Green Version]
- Bogunia-Kubik, K.; Gieryng, A.; Dlubek, D.; Lange, A. The CXCL12-3-A allele is associated with a higher mobilization yield of CD34 progenitors to the peripheral blood of healthy donors for allogeneic transplantation. Bone Marrow Transplant. 2009, 44, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Martín-Antonio, B.; Carmona, M.; Falantes, J.; Gil, E.; Baez, A.; Suarez, M.; Marín, P.; Espigado, I.; Urbano-Ispizua, A. Impact of constitutional polymorphisms in VCAM1 and CD44 on CD34 cell collection yield after administration of granulocyte colony-stimulating factor to healthy donors. Haematologica 2011, 96, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.L.; Rettig, M.P.; Motabi, I.H.; McFarland, K.; Trinkaus, K.M.; Hladnik, L.M.; Kulkarni, S.; Abboud, C.N.; Cashen, A.F.; Stockerl-Goldstein, K.E.; et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 2012, 119, 3917–3924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargetzi, M.J.; Passweg, J.; Baertschi, E.; Schoenenberger, A.; Gwerder, C.; Tichelli, A.; Burger, J.; Mingrone, W.; Herrmann, R.; Gratwohl, A.; et al. Mobilization of peripheral blood progenitor cells with vinorelbine and granulocyte colony-stimulating factor in multiple myeloma patients is reliable and cost effective. Bone Marrow Transplant. 2003, 31, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heizmann, M.; O’Meara, A.C.; Moosmann, P.R.; Heijnen, I.A.; Zuberbühler, M.; Fernandez, P. Efficient mobilization of PBSC with vinorelbine/G-CSF in patients with malignant lymphoma. Bone Marrow Transplant. 2009, 44, 75–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, A.; Friess, D.; Mansouri Taleghani, B.; Keller, P.; Mueller, B.U.; Baerlocher, G.M.; Baerlocher, G.M.; Leibundgut, K.; Pabst, T. Role of plerixafor in autologous stem cell mobilization with vinorelbine chemotherapy and granulocyte-colony stimulating factor in patients with myeloma: A phase II study (PAV-trial). Leuk. Lymphoma 2015, 56, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Seipel, K.; Novak, U.; Mueller, B.U.; Taleghani, B.M.; Leibundgut, K.; Pabst, T. Neurotoxicity of stem cell mobilization chemotherapy with vinorelbine in myeloma patients after bortezomib treatment. Leuk. Res. 2015, 39, 786–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, B.U.; Keller, S.; Seipel, K.; Mansouri Taleghani, B.; Rauch, D.; Betticher, D.; Egger, T.; Pabst, T. Stem cell mobilization chemotherapy with gemcitabine is effective and safe in myeloma patients with bortezomib-induced neurotoxicity. Leuk. Lymphoma 2017, 57, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toh, H.C.; Sun, L.; Koh, C.H.; Aw, S.E. Vinorelbine induces apoptosis and caspase-3 (CPP3) expression in leukemia and lymphoma cells: A comparison with vincristine. Leuk. Lymphoma 1998, 31, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Landini, I.; Bartolozzi, B.; Banchelli, I.; Degli Innocenti, A.; Nocentini, O.; Bernabei, P.A. In vitro activity of vinorelbine on human leukemia cells. J. Chemother. 2001, 13, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Gorin, N.C.; Labopin, M.; Czerw, T.; Pabst, T.; Blaise, D.; Dumas, P.Y.; Nemet, D.; Arcese, W.; Trisolini, S.M.; Wu, D.; et al. Autologous stem cell transplantation for adult acute myelocytic leukemia in first remission-better outcomes after busulfan and melphalan compared with busulfan and cyclophosphamide: A retrospective study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Cancer 2017, 123, 824–831. [Google Scholar] [PubMed]
- Gorin, N.C.; Labopin, M.; Blaise, D.; Dumas, P.Y.; Pabst, T.; Trisolini, S.M.; Arcese, W.; Houhou, M.; Mohty, M.; Nagler, A. Optimizing the pretransplant regimen for autologous stem cell transplantation in acute myelogenous leukemia: Better outcomes with busulfan and melphalan compared with busulfan and cyclophosphamide in high risk patients autografted in first complete remission: A study from the acute leukemia working party of the EBMT. Am. J. Hematol. 2018, 12, 1–8. [Google Scholar]
- Ferrara, F.; Pinto, A. Acute myeloid leukemia in the elderly: Current therapeutic results and perspectives for clinical research. Rev. Recent Clin. Trials. 2007, 2, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Giralt, S. Therapy for older AML patients: The role of novel agents and allogeneic stem cell transplant. J. Natl. Compr. Canc. Netw. 2008, 6, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Ustun, C.; Lazarus, H.M.; Weisdorf, D. To transplant or not: A dilemma for treatment of elderly AML patients in the twenty-first century. Bone Marrow Transplant. 2013, 48, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | AML <60 years (n = 103) | AML 60–64 years (n = 27) | AML >64 years (n = 18) | p |
|---|---|---|---|---|
| Age, median, years | 55 | 63 | 68 | <0.001 |
| Gender, male, % | 56 | 55 | 55 | n.s. |
| Hemoglobin (g/L) | 89 | 90 | 88 | n.s. |
| WBC (G/L) | 16 | 14 | 11 | n.s. |
| Peripheral blasts (%) | 39 | 38 | 38 | n.s. |
| Bone marrow blasts (%) | 68 | 66 | 65 | n.s. |
| Platelets (G/L) | 62 | 68 | 66 | n.s. |
| LDH (IU/L) | 755 | 595 | 652 | n.s. |
| FAB-M0, n (%) | 10 (10) | 2 (8) | 1 (6) | n.s. |
| M1 | 33 (31) | 9 (33) | 5 (27) | n.s. |
| M2 | 16 (16) | 3 (11) | 3 (17) | n.s. |
| M3 | 1 (1) | 0 (0) | 0 (0) | n.s. |
| M4 | 15 (15) | 3 (11) | 3 (17) | n.s. |
| M5 | 25 (24) | 9 (33) | 6 (33) | n.s. |
| MDS-/th-related | 3 (3) | 1 (4) | 0 (0) | n.s. |
| Adverse-risk, n (%) | 8 (8) | 3 (9) | 1 (6) | n.s. |
| -5 or del (5q) | 2 (2) | 1 (3) | 0 (0) | n.s. |
| Others | 3 (3) | 1 (3) | 1 (6) | n.s. |
| Complex karyotype | 3 (3) | 1 (3) | 0 (0) | n.s. |
| Intermediate-risk, n (%) | 44 (43) | 12 (42) | 9 (50) | n.s. |
| NPM1mut+FLT3-ITD | 26 (25) | 6 (24) | 4 (23) | n.s. |
| NPM1wt+FLT3-ITD | 5 (5) | 2 (6) | 1 (5) | n.s. |
| Normal karyotype | 9 (9) | 2 (6) | 2 (11) | n.s. |
| Others | 4 (4) | 2 (6) | 2 (11) | n.s. |
| Favorable risk, n (%) | 51 (49) | 14 (49) | 8 (44) | n.s. |
| t (8;21)/RUNX1-RUNX1T1 | 7 (7) | 2 (6) | 2 (11) | n.s. |
| inv (16)/CBFB-MYH11 | 11 (10) | 4 (14) | 2 (11) | n.s. |
| NPM1mut+FLT3wt | 24 (23) | 5 (19) | 3 (17) | n.s. |
| CEBPAmut | 9 (9) | 3 (10) | 1 (5) | n.s. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, B.U.; Seipel, K.; Bacher, U.; Pabst, T. Autologous Transplantation for Older Adults with AML. Cancers 2018, 10, 340. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers10090340
Mueller BU, Seipel K, Bacher U, Pabst T. Autologous Transplantation for Older Adults with AML. Cancers. 2018; 10(9):340. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers10090340
Chicago/Turabian StyleMueller, Beatrice U., Katja Seipel, Ulrike Bacher, and Thomas Pabst. 2018. "Autologous Transplantation for Older Adults with AML" Cancers 10, no. 9: 340. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers10090340





