2.1.1. CO Conversion Efficiency and Light-Off Testing
Catalytic CO oxidation was performed under constant gas composition where the concentration of CO is 3.5%, with a surplus of oxygen (20% concentration) balanced in helium to allow for the complete CO conversion. The result was obtained under controlled heating (heating rate is 10 °C/min), and subsequent normal cooling conditions (ignition/extinction) as a function of the reaction (catalyst) temperature and not the feed gas inlet temperature, as reported by other researchers in this field [
17]. In this context, we defined the ignition or light-off temperature (
Tig) as the temperature at which the CO conversion efficiency reaches 3% during heating and extinction or light-out temperature (
Text) as the temperature at which the CO conversion efficiency reaches 3% during cooling. Furthermore, we define the hysteresis as the difference between the temperatures at which the CO conversion efficiency is 50% during ignition and extinction.
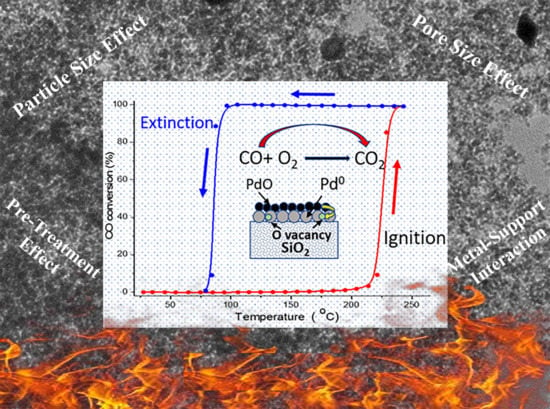
Figure 1a presents the conversion efficiency and catalyst temperature during the catalytic CO conversion test as a function of time, while
Figure 1b shows the conversion efficiency as a function of the catalyst temperature for two consecutive light-off cycles. The figures show clearly the exothermic evolution as a function of time and the hysteresis effect for oxidation reaction. During heating (ignition), three active conversion zones can be distinguished in which the reaction takes place at varying catalyst temperatures. In Zone I, when the catalyst temperature is below 214 °C, the reaction rate is slow, leading to low CO conversion as it is kinetically controlled. In Zone II, intermediate conversion zone when the temperature is above 214 °C, the reaction rate is slightly higher, leading to better CO conversions as it is controlled by internal diffusion and mass transfer limitations in the SiO
2 porous support [
18]. In Zone III, the full conversion zone when the temperature increased to above 276 °C, at which the CO conversion efficiency reaches 50%, the reaction rate is high and controlled by external (gas phase) diffusion without any mass transfer limitations.
On the other hand, during cooling (extinction), the reaction kinetics and diffusion of gas molecules play a role due to temperature differences between the high exothermic reaction heat generated at the catalyst surface and the reduced gas temperature at the reactor inlet, when the reactor is below the ignition temperature (below 214 °C). This leads to kinetic bi-stability, where the surface nanoparticles alternate between active (PdO) and in-active (Pd) states [
19]. The self-sustained oscillations and hysteresis appear during cooling of the catalyst under the flow of the reaction gas mixture. The cycling experiments for the first and second light-off were performed, where the first ignition was obtained at 88.6 °C. These results confirm that the hysteresis effect could help attain and maintain high CO conversions while oscillating between higher and lower temperatures. Upon heating/cooling, a significant hysteresis was observed at 121–296 °C. This effect can help in reducing the temperature for the second light-off for CO conversions over a Pd/SiO
2 catalyst by lowering the temperature along the extinction leg to a point below the ignition temperature as a result of the convective heat transfer associated with the temperature of the incoming gas, the CO oxidation reaction exotherm, and conduction of heat along the catalyst.
Table 1 summarizes and compares the catalytic ignition and extinction profile and hysteresis width of the Pd/SiO
2 aerogel catalyst during the first light-off (fresh catalyst) and the second light-off (heat-treated catalyst in the CO/O
2 mixture) cycles. The second light-off demonstrated higher activity compared to the first light-off cycle, as shown in
Figure 1b. The temperature of the second light-off and hysteresis width were shifted to lower values compared to the first light-off cycle. Reportedly, Pd
0 metal particles are preferably formed under a reducing CO atmosphere, whereas, under oxidizing O
2 atmosphere, Pd
2+ or PdO particles should be formed [
20]. The heating in the CO/O
2 mixture caused the CO conversion ignition temperature (
Tig) of Pd/SiO
2 to decrease from 214 to 195 °C, and the full CO conversion was achieved at (
T100) of 272 °C rather than 296 °C, while the hysteresis width decreased from 121 to 108 °C. The results might be attributed to the removal of moisture and hydrocarbons from the surface of the catalyst, increasing the metal-support interaction, as observed in XPS and XRD [
2], the formation of palladium silicide, reduction of metal hydroxide, and oxidation of metallic Pd lead to improving the active site for the CO oxidation reaction.
Furthermore, the removal of silanol groups increases the metal dispersion and the catalytic activity of the Pd/SiO
2 catalyst [
21]. Note that the conversion curve remained the same even after four cycles. The effect of heating rate on the catalytic activity, hysteresis, and ignition/extinction profile of the Pd/SiO
2 aerogel catalyst will be reported in a future manuscript.
To further investigate the catalytic activity and hysteresis behavior of the catalyst during the first and second light-off (or before and after catalytic conversion experiments), changes in the catalyst’s porosity, surface area, Pd particle size, the oxidation state of Pd, crystallography, and pore size were investigated before and after the CO oxidation reaction.
The porosity of the catalyst assumes a role in the catalytic activity and the hysteresis activity during the CO oxidation reaction. The N
2 adsorption-desorption characteristics of fresh (as prepared) Pd/SiO
2 aerogel isotherms and pore size distribution before the CO oxidation reaction was performed. The isotherms of the fresh sample with the N
2 uptake consistent with type IV (according to the IUPAC classification), and a narrow H
2 type hysteresis loop at
p/p0 > 0.75 are shown in
Figure S1, suggesting mesoporous and microporous characteristics due to capillary condensation in silica [
22]. The increment of adsorption at
p/p0 = 1.0 was caused by larger mesopores, typical in mesoporous materials [
23]. This wider hysteresis loop is known to occur when the distributions of the pore radius are wide [
24]. The effect of heat treatment in the CO/O
2 atmosphere (after the first light-off) on the morphologies, surface area, and pore volume distributions were investigated to clarify the higher catalytic activity in the second light-off observed for CO oxidation on the Pd/SiO
2 catalyst. The structural properties of both samples were shown in
Table 2 and illustrated in
Figure S1. The fresh Pd/SiO
2 aerogel catalyst shows continuous pore volume distribution with diameters between 2 and 80 nm. However, N
2 adsorption-desorption characteristics of the Pd/SiO
2 aerogel pore size distribution after CO oxidation (after the first light-off) show a pore volume with continuous distribution of pore diameters (between 2 and 60 nm). The higher temperature during the CO oxidation does not affect the integrity of the catalyst. However, the quantity of large mesopores and macropores is eliminated due to the collapse following the heat treatment, leading to a higher ratio of micropores and small mesopores, as reported by Gage et al. [
25]. BET results show that the average pore diameter of the Pd/SiO
2 aerogel slightly reduced to 15.6 nm and the pore volume slightly increased to 0.06 cm
3/g, although the surface area reduced to ~940.9 m
2/g. The decrease in the surface area could be attributed to the sintering of the large excess palladium particles outside the pores as found in electron microscopy, which will be discussed later.
To study the effect of the particles size of the catalyst on the catalytic activity and hysteresis width during the CO oxidation reaction, the TEM of the catalyst before (fresh) (
Figure 2a) and after CO oxidation (first light-off) (
Figure 2b) was performed. The presence of a large number of smaller Pd particles (2–5 nm) in the framework (marked by yellow arrows) located inside the mesoporous framework of SiO
2 aerogels homogeneously dispersed within the SiO
2 network and a few large surface particles (20–40 nm) (marked by magenta arrows). This unique texture is believed to occur due in the samples prepared by the sol-gel synthesis resulting in a better catalytic activity and effectively hindering the sintering of Pd particles [
26]. The average size estimated from over 200 particles shows a mean size of the Pd particles in as-synthesized particles as 6.1 nm.
Figure 2d,e shows the TEM micrographs of typical samples after CO oxidation. The mean size of Pd particles following the completion of the reaction was found to grow to ~7.8 nm due to the sintering, as also observed from the X-ray diffraction (XRD) analysis, and reported in a previous work [
2].
The XPS spectrum of the core level Pd 3d peaks were obtained from the samples at different points and verified by the NIST Standard XPS Database for PdO
x/Pd and Pd/SiO
x. The deconvolution of the spectrum of Pd 3d shows two spin-orbital states, 3d
5/2 and 3d
3/2 [
2]. The Pd 3d and O 1s XPS peaks located in the near-surface region of freshly prepared Pd/SiO
2 samples, at 250 °C (full CO conversion), and after catalytic CO oxidation were investigated to understand the chemical environment, palladium oxidation state, and active species before and after the CO oxidation reaction. The Pd 3d showed peaks for Pd 3d
5/2 and Pd 3d
3/2 and fitted with the mixed Pd(0) and PdO combined spectrum.
Table 3 summarizes the binding energies (BEs) of palladium Pd 3d
5/2, Pd 3d
3/2, and the binding energy (BE) of the corresponding O 1s peak [
27]. The O 1s peak was fitted with three components due to the overlap of O 1s and Pd 3P3/2 peaks following Zemlyanove et al. [
28].
Figure 3 shows Pd 3d and O 1s spectra of the reduced Pd/SiO
2 catalysts along with the deconvoluted peaks.
Figure 3a shows Pd 3d spectra for the fresh (or as prepared) catalyst with two peaks at binding energies (BE) at 334.06 and 339.3 eV assigned to the metallic palladium Pd(0) and two peaks observed at 335.8 and 341 eV assigned to Pd
2+ or PdO, respectively. This clearly indicates that most of Pd exists in the form of Pd metal with only a small fraction in the Pd
2+ form. Upon increasing the temperature to 250 °C (
Figure 3b), the obtained spectrum of the sample shows that the low energy doublet of 3d
5/2 is shifted to 335.1 eV which could be assigned to the photoemission of electrons from Pd
2+ and the high energy doublet is shifted to 336.1 eV due to the oxidation of smaller Pd particles (2–3 nm) or Pd
2+ cations within the catalyst structure [
29]. The increase in BE of Pd 3d for the catalyst annealed at 250 °C affirms the increase in formation of PdO, indicating that the surface of Pd is highly oxidized. After the CO oxidation reaction (
Figure 3c,f) the deconvolution of the Pd 3d peaks show Pd(0) and Pd
2+ (PdO) peaks with higher concentration of PdO compared to freshly prepared samples.
To analyze the change in the concentration of Pd
2+ before and after CO oxidation, we compared the areas under the Pd 3d
5/2 of Pd(0):Pd
2+ peaks before CO oxidation (
Figure 3a) to the ratio of areas of the peaks after CO oxidation (
Figure 3c). The ratio of areas under the peaks of Pd(0):Pd
2+ were 1.8:1 before and 1:1.6 after CO oxidation, respectively, indicating that the PdO concentration increases after CO oxidation, suggesting a lowering of the activation energy and confirming that the active phase for CO oxidation is PdO [
29]. The increase in surface oxygen concentration (Pd-O) after the reaction (
Figure 3f) could aid the activation of the C-O bond in the CO molecule for other oxidation reactions as reported elsewhere [
23]. This increase is also accompanied by a reduction of oxygen concentration (O-Pd-O), which suggests that Pd interacts with the SiO
2 support [
30,
31]. Oxygen in SiO
2 loses electrons resulting in a shift and bending of the Fermi level. O
2 initially diffuses to the metallic Pd surface, where it is adsorbed to form an active adsorption state. Following this, the oxygen atoms interact with the Pd atoms on the surface to form PdO.
Results obtained from the XPS analysis show that the active sites and the state of Pd can provide valuable information on the metal-support interaction in the Pd/SiO
2 catalyst through monitoring of the electronic modifications of the Pd surface before and after the CO oxidation. This observation is consistent with the XRD [
2] and TEM results. The XPS of the freshly prepared Pd/SiO
2 aerogel catalyst confirms that the Pd nanoparticles are attached to the support material through oxygen atoms of either the free silanol or siloxane groups present on the silica-network as oxygen is highly electronegative and can draw more electrons from Pd nanoparticles, resulting in higher BE for the Pd atoms in the Pd/SiO
2 catalyst.
2.1.2. Effect of Annealing Atmosphere on Catalytic Efficiency and Hysteresis of Pd/SiO2 Aerogel Catalyst
The oxidation state of Pd alone cannot explain the change of the catalytic activity and hysteresis behavior before and after the heat treatment. To examine the thermal stability and catalytic performance of the catalyst aiming at optimizing the best condition for Pd active sites, we conducted several experiments to support our results. It has been reported that the catalyst support modification can contribute to the activity and hysteresis behavior of the catalyst due to its binding to the catalyst metal. This metal–support interaction modifies both the electronic and geometric properties of the catalyst support, which influences the activity of the catalytic sites on the metal surface and enhances active sites [
23]. Reportedly, the hysteresis effect depended on the pretreatment of catalysts and was attributed to the changes in the catalyst structure for CO oxidation on partially oxidized Pd nanoparticles, where hysteresis effects were found to depend on the pretreatment of catalyst samples [
32]. Pretreatment conditions of the catalyst influence the catalytic activity, and metal-support interaction motivated by the oxidation state of metal and the nature of the reactions. The effect of the catalyst pretreatment on the hysteresis behavior was reported and attributed to the changes in the catalyst structure [
17]. Therefore, the pretreatment atmosphere is an essential factor that influences the final state of the catalyst and metal-support interaction. Oxidizing or reducing the atmosphere can yield oxide active or metallic phases depending on the temperature of the treatment.
The effect of the pretreatment atmosphere on catalytic activities, hysteresis, and ignition/extinction curves of Pd/SiO
2 aerogel catalyst was studied, and the results are summarized in
Table 4 and
Figure 4. The catalytic ignition/extinction curves and hysteresis of Pd/SiO
2 aerogel catalyst freshly prepared and annealed in different atmospheres have been plotted. It is clear that under ignition, the catalytic activities of annealed samples are higher, and the ignition temperatures are shifted to lower temperatures, which are attributed to the efficient removal of adsorbents from the surface, improving the exposure of active sites to fresh adsorbates possibly led by the decomposition of the metal complex to metal or metal oxide. However, the best activity and highest increase in the hysteresis width was observed for the sample treated in air. The results can be explained based on the gas composition of the annealing atmosphere, the reactant gas mixture, and the nature of gas used in the annealing atmosphere (reducing or oxidizing). Although, He and N
2 are inert gasses and do not affect the oxidation state of the freshly prepared Pd/SiO
2 aerogel catalyst, the thermal treatment at high temperature can affect the physio-chemical properties of the catalyst. The Pd/SiO
2-air aerogel sample showed the best catalytic activity compared to the untreated Pd/SiO
2 and Pd/SiO
2-N
2 aerogel samples, a plausible reason being that during the reaction, some of the metallic Pd nanoparticles converts to PdO as observed in XPS results, and the interfaces between Pd and PdO act as active catalytic sites. During extinction, the sample treated in N
2 gas shows an extinction temperature of 123 °C and hysteresis width of 104 °C, while the Pd/SiO
2-air aerogel sample, on the other hand, has an extinction temperature of 79 °C and a wider hysteresis width of 138 °C. This may arise from the formation of PdO in the Pd/SiO
2-air aerogel sample in the whole bulk of the catalyst during heating in the presence of excess O
2 through surface oxidation of metallic Pd preceded by diffusion of oxygen atoms from the bulk of the catalyst [
33]. During extinction, the surface PdO is reduced directly to metallic Pd. The appearance of the hysteresis loop can be associated with the slow transition from an oxygen-enriched surface and surface palladium oxide formation, present during extinction, to a CO-covered surface including Pd reduction, resulting in reversible formation of surface Pd oxide. Since Pd in the Pd/SiO
2-air aerogel sample was oxidized entirely, the reduction of Pd oxide will take a longer time than in the Pd/SiO
2 aerogel treated in N
2 samples, resulting in the broadening of the hysteresis curve [
34].
To investigate the thermal stability of Pd/SiO
2 aerogel catalyst and the surface oxidation and reduction of Pd/SiO
2, TGA was performed up to 600 °C at a heating rate of 10 °C/min in air and N
2 atmospheres, while the DSC of Pd/SiO
2 aerogel was carried out under heating (red) and cooling (black) at a heating rate of 10 °C/min in air and N
2 atmospheres up to 300 °C, which corresponds to the temperature where full CO conversion is reached. TG-DSC spectra are shown in
Figure 5. During heating, TGA and DSC studies for samples treated in an air environment showed two exothermic peaks and one endothermic peak, as shown in
Figure 5a,c. The spectra indicate clearly that the catalyst is thermally stable up to 600 °C. A weight loss of ~3 wt% occurs when the samples are heated from 50 to 100 °C due to the loss of physically adsorbed water and ethanol from the porous catalyst.
These results are consistent with the DSC results, which show an endothermic peak in the region from room temperature up to 125 °C. Furthermore, an exothermic peak accompanied by an endothermic peak between 215 and 243 °C can be attributed to the surface oxidation of palladium accompanied with the reduction of silica surface, indicative of the formation of the interface between the palladium and silica support [
35]. During the cooling ramp, as shown in
Figure 5c, two exothermic peaks at 315 and 225 °C accompanied with an endothermic peak in between, could be attributed to the reduction of the small domain PdO to Pd
0 on the surface of PdO, leading to polycrystalline particles that easily re-oxidize upon cooling due to the lack of Pd nucleation sites on the surface of the metal particles. Moreover, this could be associated with the combustion of unreacted organics such as Si–CH
3 groups from the synthesis process. This behavior was observed previously by Datye et al. on the surface of Pd/Al
2O
3 when the catalysts were heated in air [
36] and Colussi et al. at higher temperature on Pd/Al
2O
3 and Pd/CeO
2/Al
2O
3, where they reported re-dispersion of Pd on the surface of oxide and transformation between Pd and PdO [
37]. The temperature of this transformation was reported to strongly depend on the characteristics of the oxide support [
38]. The results agree with the XPS analysis and catalytic tests of Pd/SiO
2 treated in air under heating/cooling cycles. However, TGA and DSC studies for samples treated in a nitrogen environment showed only well-resolved steps,
Figure 5b,d. The major weight loss (about 5 wt%) was observed between 50 and 125 °C as evident in the DSC, as the broad endothermic peak in the curve in
Figure 5d is attributed to the loss of water from the porous catalyst [
39]. After 125 °C, the weight loss until 600 °C is attributed to the condensation of silanol groups from the surface of pristine silica aerogel, which was experimentally found to occur between 150 and 500 °C, as reported by Mueller et al. [
40]. No peaks were observed during the cooling cycle which suggest that most of Pd in the sample is in the metallic state.
It is well known that the support nature and composition of SiO
2 and pretreatment conditions (oxidation or reduction treatments) have a direct impact on the metal-support interaction and ratio of oxidized and reduced forms of the supported palladium metal. To investigate the thermal effect on the catalytic activities, hysteresis behavior, and surface oxidation and reduction of Pd/SiO
2 heated in air and N
2 atmospheres, we preformed FTIR spectroscopy of Pd/SiO
2 aerogel catalyst annealed in different atmospheres (N
2 and air) and compared it to the untreated catalysts.
Figure S2 compares FTIR spectra of freshly prepared Pd/SiO
2 aerogel catalysts and after the heat treatment at 450 °C in air and nitrogen atmospheres. Room-temperature FTIR spectra of Pd/SiO
2 samples measured in spectral range (400–4000 cm
−1) were recorded and compared to the untreated samples to determine the changes after annealing in N
2 and air atmospheres. The FTIR spectra of both samples revealed several sharp, well-defined absorption bands within the measured spectral range. The spectra show bands centered at 567, 794.5, and 1049.1 cm
−1 with a shoulder peak at 1162.9 cm
−1 corresponding to stretching vibrations of siloxane groups (Si–O–Si bonds), respectively [
41], while the peak centered at 954.5 cm
−1 corresponds to the stretching vibration of silanol groups (Si-OH) in the silica lattice suggesting the presence of a considerable amount of silanol groups on the silica surface or pores in all the samples. A small peak observed at 2987.2 cm
−1 is assigned to the vibrations of the stretching vibration of -CH
3 and -CH
2 groups indicating the presence of a small amount of Si–OC
2H
5 groups, which can be attributed to an incomplete condensation during gelation [
42]. The low-intensity peak at 3367.1 cm
−1 is assigned to -OH stretching vibrations [
43].
The FTIR spectra of the Pd/SiO
2 sample treated in air show that all peaks are shifted to a higher wavenumber compared to the untreated sample indicating the interaction between Pd and SiO
2, which can affect the formation of the Si-O-Si network as observed in Cu/SiO
2 [
44] leading to a stronger metal-support interaction. The intensity of the peak at 958.45 cm
−1 is considerably lower, while that at 794.5 cm
−1 increases indicating the formation of new Si–O–Si bonds via the reduction of Si-OH bonds as a result of the condensation reaction between Si–O and the Pd metal. This reaction could shrink the SiO
2 network, which might be responsible for decreasing the pore volume and, consequently, surface area, as observed in N
2 adsorption-desorption results suggesting that the SiO
2 framework is formed by the Si–O–Si bonds [
19]. Furthermore, the intensity of the peak at 3367.1 cm
−1, which is assigned to -OH stretching vibrations decreases, suggesting removal of the adsorbed OH or water. However, the FTIR spectra of Pd/SiO
2 sample treated in a nitrogen atmosphere showed similar spectra of the untreated sample (no shift is observed) except for reducing the intensity of the peak at 1162.9 cm
−1, which indicates the reduction of the Si-O-Si bond. The results suggest that the heat treatment in N
2 environment did not affect the interaction between Pd and the SiO
2 network.
2.1.3. Effect of Annealing Temperature on Catalytic Efficiency and Hysteresis of Pd/SiO2 Aerogel Catalyst
A further impact of annealing temperature on the catalytic activity, hysteresis, and ignition/extinction of Pd/SiO
2 treated in the air aerogel catalyst was studied by annealing some samples at 450 and 750 °C in air as summarized in
Table 5 and shown in
Figure 6. During the ignition cycle, the air-annealed catalysts demonstrated higher activity compared to the freshly prepared catalysts mainly due to the removal of moisture and hydrocarbons from the surface of the catalyst and the reduction of metal oxide to metallic Pd and improving the active site for the CO oxidation reaction. The catalyst treated at 450 °C in air shows better activity than fresh catalysts due to the removal of silanol groups, which increases the metal dispersion and the catalytic activity of Pd/SiO
2 catalyst [
21]. The results indicate that the heat treatment in air does not probably affect the Pd clusters or particles as they are pinned to the silica surface, and the diffusion of ions is difficult, preventing sintering. The Pd clusters showed resistance to sintering upon calcination to 550 °C in air, which was attributed to the confinement of Pd clusters within mesopores [
45].
The Pd/SiO
2-air aerogel catalyst initially contained a considerable amount of metal oxide; heating in air will ensure that the sample is fully oxidized at 450 °C. In studies on the catalytic activities conducted in the CO/O
2 mixture, PdO will undergo a reduction to Pd, which in-effect would prevent any Pd particle growth (due to sintering) up to 700 °C, except for outermost particles (outside the pores). Upon annealing at 750 °C, all PdO will be reduced to metallic Pd and this will lead to the growth and sintering of metallic Pd particles which would lower catalytic activity [
46]. The presence of fully oxidized Pd particles and well-defined active sites in the samples annealed at 450 °C in air ensures higher activity and lower extinction temperature. The as-prepared samples contain a considerable amount of metallic Pd, and most of the active sites are blocked, making it less active even during extinction. For samples annealed at 750 °C, the growth and sintering of the Pd particles lead to a lower activity resulting in narrower hysteresis width and lower ignition temperature compared to the sample treated at 450 °C.
Based on the experimental results, the origin of the high thermal stability and catalytic performance of Pd/SiO2 aerogel catalysts were attributed to their mesoporous structures as confirmed by the N2 adsorption-desorption isotherm, XPS, FTIR, and TEM. The results also confirmed that the catalyst structure could protect the Pd nanoparticles from sintering during the thermal treatment and catalytic CO oxidation reaction. The thermal stability of the Pd/SiO2 catalyst highly depends on the oxidative/reductive nature of the gas environment. Under the CO oxidation reaction in the oxygen atmosphere, a small amount of Pd is converted to PdO. However, under air atmosphere, the porous structure of the silica is still stable, and the formation of small Pd particles inside the SiO2 pores and on the surface increases as temperature increases.
Moreover, the absence of reducing gases in air leads to the oxidation of Pd particles, which directly impacts thermal stability and catalyst performance. The same behavior was observed under air and H
2 environment [
47]. The FTIR results confirmed that the interaction of Pd sites with -OH on the SiO
2 stabilizes the catalyst surface resulting in excellent thermal stability. Consequently, the Pd/SiO
2 catalysts showed a much more stable CO oxidation performance after annealing in air. These results agree well with recent studies, suggesting that the thermal treatment by annealing and catalytic CO oxidation at high temperatures (below 500) enhances the stability of Pd/SiO
2 catalyst under catalytic CO oxidation reactions due to its structure stability [
48].
To understand the effect of annealing temperature on the catalytic activities, hysteresis behavior, and surface oxidation and reduction of Pd/SiO
2 catalyst heated in air atmosphere at different temperatures. We performed FTIR spectroscopy of Pd/SiO
2 aerogel catalyst annealed at 450 and 750 °C in air atmospheres than the untreated catalyst.
Figure S3 shows FTIR spectra of the freshly prepared Pd/SiO
2 aerogel catalyst and after the heat treatment at 450 and 750 °C in air, respectively. The infrared absorption is similar to the discussion mentioned above in
Figure S2 with bands centered at 567, 794.5, and 1049.1 cm
−1 with a shoulder at 1162.9 cm
−1 corresponding to stretching vibrations of siloxane groups (Si–O–Si bonds), respectively. A peak centered at 954.5 cm
−1 corresponds to the stretching vibration of silanol groups (Si-OH) in the silica lattice which suggest the presence of a considerable amount of silanol groups on the silica surface or the pores in all samples. Small peaks observed at 2987.2 and 3367.1 cm
−1 are assigned to the stretching vibration of -CH
3 and -CH
2 to -OH stretching vibrations or water, as reported earlier. As the annealing temperature increases, the peak at 958.45 cm
−1 slowly disappears, the intensity of the peak at 2987.2 cm
−1 decreases, while that of the peak at 794.5 cm
−1 increases. This suggests the formation of additional Si–O–Si bonds by the condensation reaction. The peaks at 1710 and 3367.1 cm
−1 that belong to vibrations of water molecules decrease with the increasing annealing temperature due to the removal of water from the SiO
2 network structure [
23]. The results suggest that heating to 750 °C could lead to the formation of additional Si–O–Si bonds, which strengthens the SiO
2 network structure [
19]. The presence of 958.45 cm
−1, which corresponds to the stretching vibration of silanol groups (Si-OH) in the sample heated at 450 °C, enhanced the Pd-silica interaction, facilitating the dispersion of Pd particles.