Influence of Ni on Fe and Co-Fe Based Catalysts for High-Calorific Synthetic Natural Gas
Abstract
:1. Introduction
2. Results and Discussion
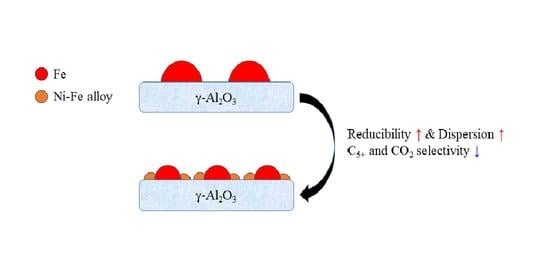
2.1. Influence of Ni on Fe Catalyst
2.2. Influence of Ni on Co-Fe Catalyst
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Characterization
3.3. Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 emissions and climate change from existing energy infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef] [Green Version]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Production of synthetic natural gas (SNG) from coal and dry biomass–A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Hong, U.G.; Seo, J.G.; Jung, J.C.; Koh, D.J.; Lim, H.; Byun, C.; Song, I.K. Methane production from carbon monoxide and hydrogen over nickel–alumina xerogel catalyst: Effect of nickel content. J. Ind. Eng. Chem. 2011, 17, 154–157. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Li, J.; Zhang, M.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Ni/Al2O3 catalysts for CO methanation: Effect of Al2O3 supports calcined at different temperatures. J. Energy Chem. 2013, 22, 919–927. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Z.; Chen, Y.; Ma, X. Syngas methanation over Ni/SiO2 catalyst prepared by ammonia-assisted impregnation. Int. J. Hydrogen Energy 2017, 42, 27073–27083. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Uba, M.; Nishida, S.; Inui, T. Application of CoMn2O3Ru catalyst to the process for producing high-calorie substitute natural gas from coke oven gas. Appl. Catal. 1989, 47, 197–208. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, H.; Choi, H.S.; Lee, D.-W.; Lee, K.-Y. Co-Mn-Ru/Al2O3 catalyst for the production of high-calorific synthetic natural gas. Korean J. Chem. Eng. 2015, 32, 2220–2226. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, D.-W.; Kim, H.; Choi, H.S.; Lee, K.-Y. Fe–Zn catalysts for the production of high-calorie synthetic natural gas. Fuel 2015, 159, 259–268. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, D.-W.; Lee, K.-Y. Production of high-calorie synthetic natural gas using copper-impregnated iron catalysts. J. Mol. Catal. A Chem. 2016, 425, 190–198. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, K.-Y. Effect of surface composition of Fe catalyst on the activity for the production of high-calorie synthetic natural gas (SNG). Korean J. Chem. Eng. 2017, 34, 320–327. [Google Scholar] [CrossRef]
- Jo, S.B.; Chae, H.J.; Kim, T.Y.; Lee, C.H.; Oh, J.U.; Kang, S.-H.; Kim, J.W.; Jeong, M.; Lee, S.C.; Kim, J.C. Selective CO hydrogenation over bimetallic Co-Fe catalysts for the production of light paraffin hydrocarbons (C2-C4): Effect of H2/CO ratio and reaction temperature. Catal. Commun. 2018, 117, 74–78. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, T.Y.; Lee, C.H.; Kang, S.-H.; Kim, J.W.; Jeong, M.; Lee, S.C.; Kim, J.C. Hybrid catalysts in a double-layered bed reactor for the production of C2–C4 paraffin hydrocarbons. Catal. Commun. 2019, 127, 29–33. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, T.Y.; Lee, C.H.; Woo, J.H.; Chae, H.J.; Kang, S.-H.; Kim, J.W.; Lee, S.C.; Kim, J.C. Selective CO Hydrogenation Over Bimetallic Co-Fe Catalysts for the Production of Light Paraffin Hydrocarbons (C2–C4): Effect of Space Velocity, Reaction Pressure and Temperature. Catalysts 2019, 9, 779. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.Y.; Jo, S.B.; Lee, C.H.; Kang, S.-H.; Kim, J.W.; Lee, S.C.; Kim, J.C. Effect of reducibility on the performance of Co-based catalysts for the production of high-calorie synthetic natural gas. Korean J. Chem. Eng. 2020, 37, 1690–1698. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jo, S.B.; Woo, J.H.; Lee, J.H.; Dhanusuraman, R.; Lee, S.C.; Kim, J.C. Investigation of Co–Fe–Al Catalysts for High-Calorific Synthetic Natural Gas Production: Pilot-Scale Synthesis of Catalysts. Catalysts 2021, 11, 105. [Google Scholar] [CrossRef]
- Lögdberg, S.; Tristantini, D.; Borg, Ø.; Ilver, L.; Gevert, B.; Järås, S.; Blekkan, E.A.; Holmen, A. Hydrocarbon production via Fischer–Tropsch synthesis from H2-poor syngas over different Fe-Co/γ-Al2O3 bimetallic catalysts. Appl. Catal. B Environ. 2009, 89, 167–182. [Google Scholar] [CrossRef]
- Griboval-Constant, A.; Butel, A.; Ordomsky, V.V.; Chernavskii, P.A.; Khodakov, A. Cobalt and iron species in alumina supported bimetallic catalysts for Fischer–Tropsch reaction. Appl. Catal. A Gen. 2014, 481, 116–126. [Google Scholar] [CrossRef]
- Kustov, A.; Frey, A.M.; Larsen, K.E.; Johannessen, T.; Nørskov, J.K.; Christensen, C.H. CO methanation over supported bimetallic Ni–Fe catalysts: From computational studies towards catalyst optimization. Appl. Catal. A Gen. 2007, 320, 98–104. [Google Scholar] [CrossRef]
- Feyzi, M.; Mirzaei, A.A.; Bozorgzadeh, H.R. Effects of preparation and operation conditions on precipitated iron nickel catalysts for Fischer-Tropsch synthesis. J. Nat. Gas Chem. 2010, 19, 341–353. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Koso, S.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Catalytic performance and characterization of Ni-Fe catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas. Appl. Catal. A Gen. 2011, 392, 248–255. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Yang, Y.; Xiang, H.; Li, Y. Study on an iron–nickel bimetallic Fischer–Tropsch synthesis catalyst. Fuel Process. Technol. 2014, 118, 117–124. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, D.; Xiao, R.; Wu, C. Methanation of syngas (H2/CO) over the different Ni-based catalysts. Fuel 2017, 189, 419–427. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Zhang, L. Promotion effect of additive Fe on Al2O3 supported Ni catalyst for CO2 methanation. Appl. Organomet. Chem. 2018, 32, e4328. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; An, K.; Zhang, S.; Liu, G.; Liu, Y. Highly Dispersed Ni–Fe Alloy Catalysts on MgAl2O4 Derived from Hydrotalcite for Direct Ethanol Synthesis from Syngas. Energy Technol. 2020, 8, 2000205. [Google Scholar] [CrossRef]
- Liang, B.; Suzuki, T.; Hamamoto, K.; Yamaguchi, T.; Fujishiro, Y.; Awano, M.; Ingram, B.J.; Cater, J.D. Effect of the adding ferrum in nickel/GDC anode-supported solid-oxide fuel cell in the intermediate temperature. Int. J. Hydrogen Energy 2011, 36, 10975–10980. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Lay, E.N. Selective methanation of carbon monoxide in hydrogen rich stream over Ni/CeO2 nanocatalysts. J. Rare Earth 2015, 33, 619–628. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Li, M.; Cui, X.; Li, Z. Catalytic performance of CO methanation over La-promoted Ni/Al2O3 catalyst in a slurry-bed reactor. Chem. Eng. J. 2017, 313, 1548–1555. [Google Scholar] [CrossRef]
- Al-Fatesh, A.; Fakeeha, A.; Khan, W.; Ibrahim, A.; He, S.; Seshan, K. Production of hydrogen by catalytic methane decomposition over alumina supported mono-, bi-and tri-metallic catalysts. Int. J. Hydrogen Energy 2016, 41, 22932–22940. [Google Scholar] [CrossRef]





| Catalyst | Metal Content (wt.%) 1 | Crystallite Size (nm) 2 | |||||
|---|---|---|---|---|---|---|---|
| Co | Fe | Ni | Co | Fe | Ni3Fe2 | Ni | |
| 20Fe | - | 20.2 | - | - | 34.5 | - | - |
| 15Fe-5Ni | - | 14.7 | 4.8 | - | 22.6 | 7.8 | - |
| 10Fe-10Ni | - | 10.2 | 9.9 | - | - | 9.6 | - |
| 20Ni | - | - | 19.8 | - | - | - | 60.6 |
| 5Co-15Fe | 5.2 | 14.1 | - | N/A | 20.2 | - | - |
| 5Co-15Fe-2Ni | 5.1 | 14.8 | 2.2 | N/A | 20.3 | - | - |
| 5Co-15Fe-5Ni | 4.9 | 14.6 | 5.1 | N/A | 8 | 6.3 | - |
| Catalyst | Reaction Condition | CO Conv (%) | Yield (%) | C1–C4 Hydrocarbon Production Rate (mL/h) | Heating Value (kcal/Nm3) 1 | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2–C4 | C5+ | CO2 | ||||||
| 5Co-15Fe | SV: 6000 mL/g/h, 300 °C, 10 bar | 91.5 ± 1.0 | 21.5 ± 0.7 | 25.8 ± 0.6 | 23.8 ± 1.6 | 20.4 ± 0.4 | 341 | 13,230 | [13] |
| SV: 4000 mL/g/h, 350 °C, 20 bar | 99.7 ± 0.1 | 31.2 ± 0.4 | 36.1 ± 0.6 | 17.6 ± 0.4 | 14.9 ± 0.2 | 323 | 13,170 | [13] | |
| 5Co-15Fe-2Ni | SV: 6000 mL/g/h, 300 °C, 10 bar | 98.3 ± 0.2 | 38.2 ± 0.2 | 21.9 ± 0.6 | 14.8 ± 0.7 | 23.2 ± 0.2 | 433 | 11,580 | This study |
| 5Co-15Fe-5Ni | SV: 6000 mL/g/h, 300 °C, 10 bar | 99.9 ± 0.1 | 51.7 ± 0.1 | 21.8 ± 0.2 | 14.1 ± 0.3 | 12.1 ± 0.2 | 530 | 11,100 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-Y.; Jo, S.; Lee, Y.; Kang, S.-H.; Kim, J.-W.; Lee, S.-C.; Kim, J.-C. Influence of Ni on Fe and Co-Fe Based Catalysts for High-Calorific Synthetic Natural Gas. Catalysts 2021, 11, 697. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11060697
Kim T-Y, Jo S, Lee Y, Kang S-H, Kim J-W, Lee S-C, Kim J-C. Influence of Ni on Fe and Co-Fe Based Catalysts for High-Calorific Synthetic Natural Gas. Catalysts. 2021; 11(6):697. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11060697
Chicago/Turabian StyleKim, Tae-Young, Seongbin Jo, Yeji Lee, Suk-Hwan Kang, Joon-Woo Kim, Soo-Chool Lee, and Jae-Chang Kim. 2021. "Influence of Ni on Fe and Co-Fe Based Catalysts for High-Calorific Synthetic Natural Gas" Catalysts 11, no. 6: 697. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11060697