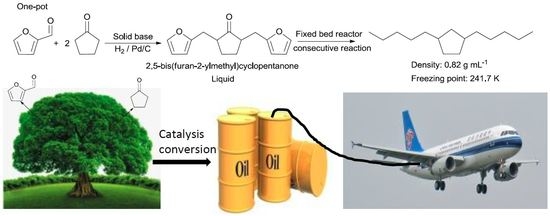
Synthesis of Diesel and Jet Fuel Range Cycloalkanes with Cyclopentanone and Furfural
Abstract
:1. Introduction
2. Results and Discussion
2.1. Aldol Condensation
2.2. One Pot Aldol Condensation/Hydrogenation
2.3. Hydrodeoxygenation (HDO)
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Aldol Condensation
3.3. One Pot Aldol Condensation
3.4. Hydrodeoxygenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Harvey, B.G.; Wright, M.E.; Quintana, R.L. High-Density Renewable Fuels Based on the Selective Dimerization of Pinenes. Energy Fuels 2010, 24, 267–273. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Zhao, C.; Kou, Y.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Highly Selective Catalytic Conversion of Phenolic Bio-Oil to Alkanes. Angew. Chem. 2009, 121, 4047–4050. [Google Scholar] [CrossRef]
- Bond, J.Q.; Upadhye, A.A.; Olcay, H.; Tompsett, G.A.; Jae, J.; Xing, R.; Alonso, D.M.; Wang, D.; Zhang, T.; Kumar, R.; et al. Production of renewable jet fuel range alkanes and commodity chemicals from integrated catalytic processing of biomass. Energy Environ. Sci. 2014, 7, 1500–1523. [Google Scholar] [CrossRef]
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel Strategies for the Production of Fuels, Lubricants, and Chemicals from Biomass. Accounts Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Wang, X.; Rinaldi, R. A Route for Lignin and Bio-Oil Conversion: Dehydroxylation of Phenols into Arenes by Catalytic Tandem Reactions. Angew. Chem. 2013, 125, 11713–11717. [Google Scholar] [CrossRef]
- Zan, Y.; Miao, G.; Wang, H.; Kong, L.; Ding, Y.; Sun, Y. Revealing the roles of components in glucose selective hydrogenation into 1,2-propanediol and ethylene glycol over Ni-MnO-ZnO catalysts. J. Energy Chem. 2019, 38, 15–19. [Google Scholar] [CrossRef]
- Fang, S.; Cui, Z.; Zhu, Y.; Wang, C.; Bai, J.; Zhang, X.; Xu, Y.; Liu, Q.; Chen, L.; Zhang, Q.; et al. In situ synthesis of biomass-derived Ni/C catalyst by self-reduction for the hydrogenation of levulinic acid to γ-valerolactone. J. Energy Chem. 2019, 37, 204–214. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gartner, C.A.; Dumesic, J.A.; Baldauf, S.L. Catalytic Conversion of Biomass to Monofunctional Hydrocarbons and Targeted Liquid-Fuel Classes. Science 2008, 322, 417–421. [Google Scholar] [CrossRef]
- Huber, G.W. Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Subrahmanyam, A.V.; Olcay, H.; Qi, W.; Van Walsum, G.P.; Pendse, H.; Huber, G.W. Production of jet and diesel fuel range alkanes from waste hemicellulose-derived aqueous solutions. Green Chem. 2010, 12, 1933. [Google Scholar] [CrossRef]
- Corma, A.; De La Torre, O.; Renz, M.; Villandier, N. Production of High-Quality Diesel from Biomass Waste Products. Angew. Chem. 2011, 50, 2375–2378. [Google Scholar] [CrossRef]
- Corma, A.; Arias, K.S.; Climent, M.J.; Iborra, S. Synthesis of high quality alkyl naphthenic kerosene by reacting an oil refinery with a biomass refinery stream. Energy Environ. Sci. 2015, 8, 317–331. [Google Scholar]
- Xia, Q.-N.; Cuan, Q.; Liu, X.-H.; Gong, X.-Q.; Lu, G.-Z.; Wang, Y.-Q. Pd/NbOPO 4 Multifunctional Catalyst for the Direct Production of Liquid Alkanes from Aldol Adducts of Furans. Angew. Chem. 2014, 53, 9755–9760. [Google Scholar] [CrossRef]
- Sacia, E.R.; Balakrishnan, M.; Deaner, M.H.; Goulas, K.A.; Toste, F.D.; Bell, A.T. Highly Selective Condensation of Biomass-Derived Methyl Ketones as a Source of Aviation Fuel. ChemSusChem 2015, 8, 1726–1736. [Google Scholar] [CrossRef]
- Nie, G.; Zhang, X.; Han, P.; Xie, J.; Pan, L.; Wang, L.; Zou, J.-J. Lignin-derived multi-cyclic high density biofuel by alkylation and hydrogenated intramolecular cyclization. Chem. Eng. Sci. 2017, 158, 64–69. [Google Scholar] [CrossRef]
- Tang, H.; Chen, F.; Li, G.; Yang, X.; Hu, Y.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T.; Li, N. Synthesis of jet fuel additive with cyclopentanone. J. Energy Chem. 2019, 29, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Nie, G.; Zhang, X.; Pan, L.; Wang, M.; Zou, J.-J. One-pot production of branched decalins as high-density jet fuel from monocyclic alkanes and alcohols. Chem. Eng. Sci. 2018, 180, 64–69. [Google Scholar] [CrossRef]
- Zhao, C.; Camaioni, D.M.; Lercher, J.A. Selective catalytic hydroalkylation and deoxygenation of substituted phenols to bicycloalkanes. J. Catal. 2012, 288, 92–103. [Google Scholar] [CrossRef]
- Li, H.; Riisager, A.; Saravanamurugan, S.; Pandey, A.; Sangwan, R.S.; Yang, S.; Luque, R. Carbon-Increasing Catalytic Strategies for Upgrading Biomass into Energy-Intensive Fuels and Chemicals. ACS Catal. 2017, 8, 148–187. [Google Scholar] [CrossRef]
- Jing, Y.; Xia, Q.; Xie, J.; Liu, X.; Guo, Y.; Zou, J.-J.; Wang, Y. Correction to Robinson Annulation-Directed Synthesis of Jet-Fuel-Ranged Alkylcyclohexanes from Biomass-Derived Chemicals. ACS Catal. 2018, 8, 3280–3285. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Solvent-Free Synthesis of C 10 and C 11 Branched Alkanes from Furfural and Methyl Isobutyl Ketone. ChemSusChem 2013, 6, 1149–1152. [Google Scholar] [CrossRef]
- Chen, F.; Li, N.; Li, S.; Yang, J.; Liu, F.; Wang, W.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Solvent-free synthesis of C9 and C10 branched alkanes with furfural and 3-pentanone from lignocellulose. Catal. Commun. 2015, 59, 229–232. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, S.; Wang, W.; Li, L.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of diesel and jet fuel range alkanes with furfural and ketones from lignocellulose under solvent free conditions. Green Chem. 2014, 16, 4879–4884. [Google Scholar] [CrossRef]
- Jing, Y.; Xin, Y.; Guo, Y.; Liu, X.; Wang, Y. Highly efficient Nb2O5 catalyst for aldol condensation of biomass-derived carbonyl molecules to fuel precursors. Chin. J. Catal. 2019, 40, 1168–1177. [Google Scholar] [CrossRef]
- Li, C.; Ding, D.; Xia, Q.; Liu, X.; Wang, Y. Conversion of raw lignocellulosic biomass into branched long-chain alkanes through three tandem steps. ChemSusChem 2016, 9, 1712–1718. [Google Scholar] [CrossRef]
- Xu, J.; Li, N.; Yang, X.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of Diesel and Jet Fuel Range Alkanes with Furfural and Angelica Lactone. ACS Catal. 2017, 7, 5880–5886. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K.; Vávra, I.; Soták, T.; Dobročka, E.; Mičušík, M. Carbon supported Pd–Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl. Catal. B Environ. 2016, 181, 210–219. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Z.; Huang, Y.; Lu, F.; Wang, F.; Gao, J.; Xu, J. Conversion of furfural into cyclopentanone over Ni–Cu bimetallic catalysts. Green Chem. 2013, 15, 1932–1940. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Liu, D.; Mu, X.; Chen, X.; Shi, Y. Selective Conversion of Furfural to Cyclopentanone or Cyclopentanol Using Co-Ni Catalyst in Water. Catalysts 2018, 8, 193. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Sheng, X.; Xu, Q.; Wang, X.; Li, N.; Jia, H.; Shi, H.; Niu, M.; Zhang, J.; Ping, Q. Waste Seashells as a Highly Active Catalyst for Cyclopentanone Self-Aldol Condensation. Catalysts 2019, 9, 661. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable high-density fuels using cyclopentanone derived from lignocellulose. Chem. Commun. 2014, 50, 2572–2574. [Google Scholar] [CrossRef]
- Sheng, X.; Li, G.; Wang, W.; Cong, Y.; Huber, G.W.; Zhang, T. Dual-bed Catalyst System for the Direct Synthesis of High Density Aviation Fuel with Cyclopentanone from Lignocellulose. AIChE J. 2016, 62, 2754–2761. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Li, G.; Li, S.; Wang, W.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of Renewable High-Density Fuel with Cyclopentanone Derived from Hemicellulose. ACS Sustain. Chem. Eng. 2017, 5, 1812–1817. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K.; Liptaj, T.; Stolcova, M.; Prónayová, N.; Soták, T. Cyclopentanone: A raw material for production of C15 and C17 fuel precursors. Biomass Bioenergy 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Wang, W.; Ji, X.; Ge, H.; Li, Z.; Tian, G.; Shao, X.; Zhang, Q. Synthesis of C 15 and C 10 fuel precursors with cyclopentanone and furfural derived from hemicellulose. RSC Adv. 2017, 7, 16901–16907. [Google Scholar] [CrossRef]
- Deng, Q.; Xu, J.; Han, P.; Pan, L.; Wang, L.; Zhang, X.; Zou, J.-J. Efficient synthesis of high-density aviation biofuel via solvent-free aldol condensation of cyclic ketones and furanic aldehydes. Fuel Process. Technol. 2016, 148, 361–366. [Google Scholar] [CrossRef]
- Ao, L.; Zhao, W.; Guan, Y.-S.; Wang, D.-K.; Liu, K.-S.; Guo, T.-T.; Fan, X.; Wei, X.-Y. Efficient synthesis of C15 fuel precursor by heterogeneously catalyzed aldol-condensation of furfural with cyclopentanone. RSC Adv. 2019, 9, 3661–3668. [Google Scholar] [CrossRef] [Green Version]
- Hronec, M.; Fulajtarová, K.; Liptaj, T.; Prónayová, N.; Soták, T. Bio-derived fuel additives from furfural and cyclopentanone. Fuel Process. Technol. 2015, 138, 564–569. [Google Scholar] [CrossRef]










| Catalysis | Base Sites Amount (mmol g−1) |
|---|---|
| CaO | 0.16 |
| LiAl-HT | 0.15 |
| MgAl-HT | 0.12 |
| MgO | 0.04 |
| CeO2 | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Sun, S.; Han, F.; Li, G.; Shao, X.; Li, N. Synthesis of Diesel and Jet Fuel Range Cycloalkanes with Cyclopentanone and Furfural. Catalysts 2019, 9, 886. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9110886
Wang W, Sun S, Han F, Li G, Shao X, Li N. Synthesis of Diesel and Jet Fuel Range Cycloalkanes with Cyclopentanone and Furfural. Catalysts. 2019; 9(11):886. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9110886
Chicago/Turabian StyleWang, Wei, Shaoying Sun, Fengan Han, Guangyi Li, Xianzhao Shao, and Ning Li. 2019. "Synthesis of Diesel and Jet Fuel Range Cycloalkanes with Cyclopentanone and Furfural" Catalysts 9, no. 11: 886. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9110886