Syntheses, Structures, and Physical Properties of Neutral Gold Dithiolate Complex, [Au(etdt)2]·THF
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis of nBu4N·[Au(etdt)2] (1)
2.3. Electrochemical Synthesis of [Au(etdt)2]·THF (2)
2.4. Crystal Structure Determination of 1 and 2
2.5. Electrical Resistivity Measurements of 2
2.6. Magnetic Measurements of 2
2.7. Molecular Orbital, Band Structure, and Density of State Calculations of 2
3. Results and Discussion
3.1. Crystal Structure of Monoanion Gold Complex 1
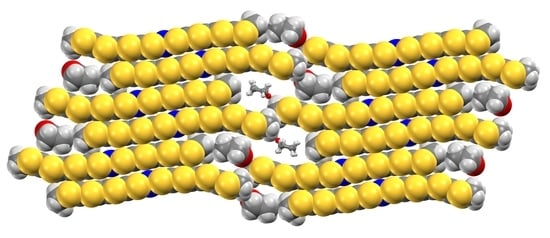
3.2. Crystal Structure of Neutral Gold Complex 2
3.3. Electrical Properties of Neutral Gold Complex 2
3.4. Magnetic Susceptibility of Neutral Gold Complex 2
3.5. Electronic Structures and Band Structure Calculations of Neutral Gold Complex 2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferraris, J.; Cowan, D.O.; Walatka, V.; Perlstein, J.H. Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc. 1973, 95, 948–949. [Google Scholar] [CrossRef]
- Jérome, D.; Mazaud, A.; Ribault, M.; Bechgaard, K.J. Superconductivity in a synthetic organic conductor(TMTSF)2PF 6. Phys. Lett. 1980, 41, 95–98. [Google Scholar]
- Kobayashi, H.; Tomita, H.; Naito, T.; Kobayashi, A.; Sakai, F.; Watanabe, T.; Cassoux, P. New BETS Conductors with Magnetic Anions (BETS = bis(ethylenedithio)tetraselenafulvalene). J. Am. Chem. Soc. 1996, 118, 368–377. [Google Scholar] [CrossRef]
- Uji, S.; Shinagawa, H.; Terashima, T.; Yakabe, T.; Terai, Y.; Tokumoto, M.; Kobayashi, A.; Tanaka, H.; Kobayashi, H. Magnetic-field-induced superconductivity in a two-dimensional organic conductor. Nature 2001, 410, 908–910. [Google Scholar] [CrossRef]
- Tanaka, H.; Okano, Y.; Kobayashi, H.; Suzuki, W.; Kobayashi, A. Three-dimenstional synthetic metallic crystal composed of single-component molecules. Science 2001, 291, 285–287. [Google Scholar] [CrossRef]
- Kobayashi, A.; Fujiwara, E.; Kobayashi, H. Single-Component Molecular Metals with Extended-TTF Dithiolate Ligands. Chem. Rev. 2004, 104, 5243–5264. [Google Scholar] [CrossRef]
- Tanaka, H.; Tokumoto, M.; Ishibashi, S.; Graf, D.; Choi, E.S.; Brooks, J.S.; Yasuzuka, S.; Okano, Y.; Kobayashi, H.; Kobayashi, A. Observation of Three-Dimensional Fermi Surfaces in a Single-Component Molecular Metal, [Ni(tmdt)2]. J. Am. Chem. Soc. 2004, 126, 10518–10519. [Google Scholar] [CrossRef]
- Rovira, C.; Novoa, J.J.; Mozos, J.-L.; Ordejón, P.; Canadell, E. First-principles study of the neutral molecular metalNi(tmdt)2. Phys. Rev. B 2002, 65, 081104. [Google Scholar] [CrossRef] [Green Version]
- Le Gal, Y.; Roisnel, T.; Auban-Senzier, P.; Bellec, N.; Íñiguez, J.; Canadell, E.; Lorcy, D. Stable Metallic State of a Neutral-Radical Single-Component Conductor at Ambient Pressure. J. Am. Chem. Soc. 2018, 140, 6998–7004. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Terauchi, T.; Sumi, S.; Matsushita, Y. Carrier generation and electronic properties of a single-component pure organic metal. Nat. Mater. 2017, 16, 109–114. [Google Scholar] [CrossRef]
- Zhou, B.; Kobayashi, A.; Okano, Y.; Nakashima, T.; Aoyagi, S.; Nishibori, E.; Sakata, M.; Tokumoto, M.; Kobayashi, H. Single-Component Molecular Conductor [Pt(tmdt)2] (tmdt = trimethylenetetrathiafulvalenedithiolate)—An Advanced Molecular Metal Exhibiting High Metallicity. Adv. Mater. 2009, 21, 3596–3600. [Google Scholar] [CrossRef]
- Takagi, R.; Miyagawa, K.; Yoshimura, M.; Gangi, H.; Kanoda, K.; Zhou, B.; Idobata, Y.; Kobayashi, A. Magnetochiral nonreciprocity of volume spin wave propagation in chiral-lattice ferromagnets. Phys. Rev. B 2016, 93, 024403. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, W.; Fujiwara, E.; Kobayashi, A.; Fujishiro, Y.; Nishibori, E.; Takata, M.; Sakata, M.; Fujiwara, H.; Kobayashi, H. Highly Conducting Crystals Based on Single-Component Gold Complexes with Extended-TTF Dithiolate Ligands. J. Am. Chem. Soc. 2003, 125, 1486–1487. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Miyagawa, K.; Kanoda, K.; Shimamura, M.; Zhou, B.; Kobayashi, A.; Kobayashi, H. NMR Evidence for Antiferromagnetic Transition in the Single-Component Molecular Conductor, [Au(tmdt)2] at 110 K. J. Phys. Soc. Jpn. 2008, 77, 053706. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Yajima, H.; Kobayashi, A.; Okano, Y.; Tanaka, H.; Kumashiro, T.; Nishibori, E.; Sawa, H.; Kobayashi, H. Single-Component Molecular Conductor [Cu(tmdt)2] Containing an Antiferromagnetic Heisenberg Chain. Inorg. Chem. 2010, 49, 6740–6747. [Google Scholar] [CrossRef]
- Takagi, R.; Hamai, T.; Gangi, H.; Miyagawa, K.; Zhou, B.; Kobayashi, A.; Kanoda, K. Single-component molecular material hosting antiferromagnetic and spin-gapped Mott subsystems. Phys. Rev. B 2017, 95, 094420. [Google Scholar] [CrossRef] [Green Version]
- Ogura, S.; Idobata, Y.; Zhou, B.; Kobayashi, A.; Takagi, R.; Miyagawa, K.; Kanoda, K.; Kasai, H.; Nishibori, E.; Satoko, C.; et al. Antiferromagnetic Ordering in the Single-Component Molecular Conductor [Pd(tmdt)2]. Inorg. Chem. 2016, 55, 7709–7716. [Google Scholar] [CrossRef]
- Takagi, R.; Sari, D.P.; Mohd-Tajudin, S.S.; Ashi, R.; Watanabe, I.; Ishibashi, S.; Miyagawa, K.; Ogura, S.; Zhou, B.; Kobayashi, A.; et al. Antiferromagnetic Mott insulating state in the single-component molecular material Pd(tmdt)2. Phys. Rev. B 2017, 96, 214432. [Google Scholar] [CrossRef]
- Kobayashi, A.; Tanaka, H.; Kumasaki, M.; Torii, H.; Narymbetov, B.; Adachi, T. Origin of the High Electrical Conductivity of Neutral [Ni(ptdt)2] (ptdt2−= propylenedithiotetrathiafulvalenedithiolate): A Route to Neutral Molecular Metal. J. Am. Chem. Soc. 1999, 121, 10763–10771. [Google Scholar] [CrossRef]
- Zhou, B.; Yajima, H.; Idobata, Y.; Kobayashi, A.; Kobayashi, T.; Nishibori, E.; Sawa, H.; Kobayashi, H. Single-component Layered Molecular Conductor, [Au(ptdt)2]. Chem. Lett. 2012, 41, 154–156. [Google Scholar] [CrossRef]
- Sasa, M.; Fujiwara, E.; Kobayashi, A.; Ishibashi, S.; Terakura, K.; Okano, Y.; Fujiwara, H.; Kobayashi, H. Crystal structures and physical properties of single-component molecular conductors consisting of nickel and gold complexes with bis(trifluoromethyl)tetrathiafulvalenedithiolate ligands. J. Mater. Chem. 2005, 15, 155–163. [Google Scholar] [CrossRef]
- Zhou, B.; Ogura, S.; Liu, Q.Z.; Kasai, H.; Nishibori, E.; Kobayashi, A. A Single-component Molecular Conductor with Metal–Metal Bonding, [Pd(hfdt)2] (hfdt= bis(trifluoromethyl)tetrathiafulvalenedithiolate). Chem. Lett. 2016, 45, 303–305. [Google Scholar] [CrossRef]
- Cui, H.; Kobayashi, H.; Ishibashi, S.; Sasa, M.; Iwase, F.; Kato, R.; Kobayashi, A. A Single-Component Molecular Superconductor. J. Am. Chem. Soc. 2014, 136, 7619–7622. [Google Scholar] [CrossRef]
- Zhou, B.; Ishibashi, S.; Ishii, T.; Sekine, T.; Takehara, R.; Miyagawa, K.; Kanoda, K.; Nishibori, E.; Kobayashi, A. Single-component molecular conductor [Pt(dmdt)2]—A three-dimensional ambient-pressure molecular Dirac electron system. Chem. Commun. 2019, 55, 3327–3330. [Google Scholar] [CrossRef]
- Kajita, K.; Nishio, Y.; Tajima, N.; Suzumura, Y.; Kobayashi, A. Molecular Dirac Fermion Systems—Theoretical and Experimental Approaches—. J. Phys. Soc. Jpn. 2014, 83, 072002. [Google Scholar] [CrossRef]
- Kajita, K.; Nishio, Y.; Moriyama, S.; Sasaki, W.; Kato, R.; Kobayashi, H.; Kobayashi, A. New organic superconductors K- and θ-(BEDT-TTF)2I3: Transport property. Solid State Commun. 1987, 64, 1279–1284. [Google Scholar] [CrossRef]
- Yagubskiĭ, É.B.; Shchegolev, I.F.; Laukhin, V.N.; Kononovich, P.A.; Karatsovnik, M.V.; Zvarykina, A.V.; Buravov, L.I. Coexistence of different superconducting phases with transition temperatures between 1.5 and 7 K in the (BEDT-TTF)- I−3 system. J. Exp. Theor. Phys. 1984, 39, 12–16. [Google Scholar]
- Nakano, M.; Kuroda, A.; Matsubayashi, G.-E. Extended bisdithiolene metal complexes: Preparation and electrical conductivities of [M(C8H4S8)2] anion complexes (M = Ni(II), Pt(II), Au(III)). Inorg. Chim. Acta 1997, 254, 189–193. [Google Scholar] [CrossRef]
- Kubo, K.; Nakano, M.; Tamura, H.; Matsubayashi, G.-E.; Nakamoto, M. Preparation and oxidation of polarized Au(III) complexes having both the C-deprotonated-2-phenylpyridine (ppy) and a sulfur-rich dithiolate ligand and X-ray crystal structure of [Au(η2-C,N-ppy)(η2-S,S-C8H4S8)]·0.5DMF. J. Organomet. Chem. 2003, 669, 141–148. [Google Scholar] [CrossRef]
- Binet, L.; Fabre, J.M.; Montginoul, C.; Simonsen, K.B.; Becher, J. Preparation and chemistry of new unsymmetrically substituted tetrachalcogenofulvalenes bearing CN(CH2)2X and HO(CH2)2X groups (X = S or Se). J. Chem. Soc. Perkin Trans. 1 1996, 783–788. [Google Scholar] [CrossRef]
- Braunstein, P.; Clark, R.J.H. The preparation, properties, and vibrational spectra of complexes containing the AuCl2−, AuBr2−, and AuI2− ions. J. Chem. Soc. Dalton Trans. 1973, 1845–1848. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history ofSHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Hursthouse, M.B.; Caulfield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W.; Ducasse, L.; et al. Superconducting and Semiconducting Magnetic Charge Transfer Salts: (BEDT-TTF)4AFe(C2O4)3.cntdot.C6H5CN (A = H2O, K, NH4). J. Am. Chem. Soc. 1995, 117, 12209–12217. [Google Scholar] [CrossRef]








| 1 | 2 | |
|---|---|---|
| Empirical Formula | C32H44AuNS16 | C20H16AuOS16 |
| Formula Weight | 1152.61 | 982.25 |
| Crystal System | monoclinic | triclinic |
| Space Group | P21/c (#14) | P-1 (#2) |
| a/Å | 20.7276 (5) | 6.4340 (3) |
| b/Å | 16.6751 (4) | 8.6985 (4) |
| c/Å | 12.7080 (3) | 28.8338 (13) |
| α/° | 90 | 84.332 (4) |
| β/° | 90.421 (2) | 87.668 (4) |
| γ/° | 90 | 69.599 (4) |
| V/Å3 | 4392.21 (18) | 1505.07 (13) |
| Z | 4 | 2 |
| Dcalc/g·cm−3 | 1.743 | 2.167 |
| Temp./K | 173 | 298 (R.T.) |
| F (000) | 2312 | 958 |
| μ/mm−1 | 4.137 | 6.018 |
| Reflections Collected | 100,597 | 11,123 |
| Independent Reflections | 10,156 | 5934 |
| Parameters | 455 | 318 |
| R1 (I > 2σ) 1 | 0.037 | 0.051 |
| wR2 (all data) 2 | 0.074 | 0.138 |
| GOF | 1.013 | 1.104 |
| CCDC | 2,036,560 | 2,036,561 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, K.; Zhou, B.; Idobata, Y.; Kamebuchi, H.; Kobayashi, A. Syntheses, Structures, and Physical Properties of Neutral Gold Dithiolate Complex, [Au(etdt)2]·THF. Crystals 2020, 10, 1001. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111001
Sakaguchi K, Zhou B, Idobata Y, Kamebuchi H, Kobayashi A. Syntheses, Structures, and Physical Properties of Neutral Gold Dithiolate Complex, [Au(etdt)2]·THF. Crystals. 2020; 10(11):1001. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111001
Chicago/Turabian StyleSakaguchi, Kazuha, Biao Zhou, Yuki Idobata, Hajime Kamebuchi, and Akiko Kobayashi. 2020. "Syntheses, Structures, and Physical Properties of Neutral Gold Dithiolate Complex, [Au(etdt)2]·THF" Crystals 10, no. 11: 1001. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10111001