Observation of a New Polyhalide Phase in Ag-Cl2 System at High Pressure
Abstract
:1. Introduction
2. Materials and Methods
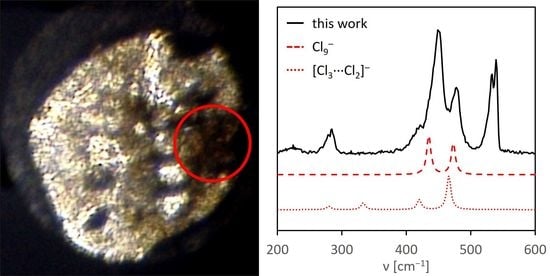
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grochala, W. Silverland: The Realm of Compounds of Divalent Silver—And Why They are Interesting. J. Supercond. Nov. Magn. 2017, 31, 737–752. [Google Scholar] [CrossRef]
- Gawraczyński, J.; Kurzydłowski, D.; Ewings, R.A.; Bandaru, S.; Gadomski, W.; Mazej, Z.; Ruani, G.; Bergenti, I.; Jaroń, T.; Ozarowski, A.; et al. Silver route to cuprate analogs. Proc. Natl. Acad. Sci. USA 2019, 116, 1495–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazej, Z.; Kurzydłowski, D.; Grochala, W. Unique Silver(II) Fluorides: The Emerging Electronic and Magnetic Materials. In Photonic and Electronic Properties of Fluoride Materials: Progress in Fluorine Science Series; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–260. ISBN 9780128017951. [Google Scholar]
- Nilges, T.; Nilges, S.; Pfitzner, A.; Doert, T.; Böttcher, P. Structure−Property Relations and Diffusion Pathways of the Silver Ion Conductor Ag5Te2Cl. Chem. Mater. 2004, 16, 806–812. [Google Scholar] [CrossRef]
- Adamczyk, W.; Połczyński, P.; Mika, A.; Jaroń, T.; Mazej, Z.; Fijalkowski, K.J.; Jurczakowski, R.; Grochala, W. New Ag(F1−XClx) phases for energy storage applications. J. Fluor. Chem. 2015, 174, 22–29. [Google Scholar] [CrossRef]
- Werner, W.; Strähle, J. The Crystal Structure of Silver Tetrachloroaurate(III) AgAuCl4 and Its Crystal Chemical Relationship to the Rubidium Tetrachloroaurate(III) RbAuCl4. Z. Nat. B 1977, 32, 741–744. [Google Scholar] [CrossRef]
- Knies, M.; Anh, M.L.; Keßler, U.; Ruck, M. Crystal structures of the tetrachloridoaluminates(III) of rubidium(I), silver(I), and lead(II). Z. Nat. B 2020, 75, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Gaebell, H.-C.; Meyer, G.; Hoppe, R. Über Chloroargentate(I): CsAgCl2 und schwarzes CsAgCl2+X. Z. Anorg. Allg. Chem. 1983, 497, 199–205. [Google Scholar] [CrossRef]
- Hull, S.; Berastegui, P. Crystal structures and ionic conductivities of ternary derivatives of the silver and copper monohalides—II: Ordered phases within the (AgX)x−(MX)1−X and (CuX)x−(MX)1−x (M = K, Rb and Cs; X = Cl, Br and I) systems. J. Solid State Chem. 2004, 177, 3156–3173. [Google Scholar] [CrossRef]
- Hasselgren, C.; Jagner, S. Dirubidiumcatena-poly[dichloroargentate(I)-μ-chloro]. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 1208–1210. [Google Scholar] [CrossRef]
- Bill, J.; Lerch, K.; Laqua, W. Cs2AgIAgIIICl6. Eine gemischtvalente Verbindung mit dreiwertigem Silber. Z. Anorg. Allg. Chem. 1990, 589, 7–11. [Google Scholar] [CrossRef]
- Allen, J.; Scanlon, D.; Watson, G.W. Electronic structure of mixed-valence silver oxide AgO from hybrid density-functional theory. Phys. Rev. B 2010, 81, 1–4. [Google Scholar] [CrossRef]
- McMillan, J. Magnetic properties and crystalline structure of AgO. J. Inorg. Nucl. Chem. 1960, 13, 28–31. [Google Scholar] [CrossRef]
- Grzelak, A.; Gawraczyński, J.; Jaroń, T.; Somayazulu, M.; Derzsi, M.; Struzhkin, V.; Grochala, W. Persistence of Mixed and Non-intermediate Valence in the High-Pressure Structure of Silver(I,III) Oxide, AgO: A Combined Raman, X-ray Diffraction (XRD), and Density Functional Theory (DFT) Study. Inorg. Chem. 2017, 56, 5804–5812. [Google Scholar] [CrossRef]
- Derzsi, M.; Grzelak, A.; Kondratiuk, P.; Tokár, K.; Grochala, W. Quest for Compounds at the Verge of Charge Transfer Instabilities: The Case of Silver(II) Chloride. Crystals 2019, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Derzsi, M.; Uhliar, M.; Tokár, K. Ag6Cl4: The first silver chloride with rare Ag6 clusters from an ab initio study. Chem. Commun. 2021, 57, 10186–10189. [Google Scholar] [CrossRef]
- Zhang, W.; Oganov, A.R.; Goncharov, A.F.; Zhu, Q.; Boulfelfel, S.E.; Lyakhov, A.O.; Stavrou, E.; Somayazulu, M.; Prakapenka, V.B.; Konôpková, Z. Unexpected Stable Stoichiometries of Sodium Chlorides. Science 2013, 342, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Bu, H.; Zhao, M.; Zhou, H.; Du, Y. Unusual electronic and mechanical properties of sodium chlorides at high pressures. Phys. Lett. A 2016, 380, 1556–1561. [Google Scholar] [CrossRef]
- Zhang, W.; Oganov, A.R.; Zhu, Q.; Lobanov, S.; Stavrou, E.; Goncharov, A. Stability of numerous novel potassium chlorides at high pressure. Sci. Rep. 2016, 6, 26265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Sonnenberg, K.; Mann, L.; Redeker, F.A.; Schmidt, B.; Riedel, S. Polyhalogen and Polyinterhalogen Anions from Fluorine to Iodine. Angew. Chem. Int. Ed. 2020, 59, 5464–5493. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Wang, Y.; Dewaele, A.; Wu, C.; Fratanduono, D.E.; Eggert, J.; Klotz, S.; Dziubek, K.F.; Loubeyre, P.; Fat’Yanov, O.V.; et al. Toward an international practical pressure scale: A proposal for an IPPS ruby gauge (IPPS-Ruby2020). High Press. Res. 2020, 40, 299–314. [Google Scholar] [CrossRef]
- Akahama, Y.; Kawamura, H. Pressure calibration of diamond anvil Raman gauge to 410 GPa. J. Phys. Conf. Ser. 2010, 215, 012195. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initiomolecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initiomolecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes forab initiototal-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, L.; Svitlyk, V.; Garbarino, G.; Sifre, D.; Mezouar, M. Structure of solid chlorine at 1.45 GPa. Z. Krist. Cryst. Mater. 2019, 234, 277–280. [Google Scholar] [CrossRef]
- von der Osten, W. Polarized Raman spectra of silver halide crystals. Phys. Rev. B 1974, 9, 789–793. [Google Scholar] [CrossRef]
- Martina, I.; Wiesinger, R.; Schreiner, M. Micro-Raman Characterisation of Silver Corrosion Products: Instrumental Set Up and Reference. e-Preserv. Sci. 2012, 9, 1–8. [Google Scholar]
- Hull, S.; Keen, D.A. Pressure-induced phase transitions in AgCl, AgBr, and AgI. Phys. Rev. B 1999, 59, 750–761. [Google Scholar] [CrossRef]
- Grzelak, A.; Gawraczyński, J.; Jaroń, T.; Kurzydłowski, D.; Budzianowski, A.; Mazej, Z.; Leszczyński, P.J.; Prakapenka, V.B.; Derzsi, M.; Struzhkin, V.V.; et al. High-Pressure Behavior of Silver Fluorides up to 40 GPa. Inorg. Chem. 2017, 56, 14651–14661. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, P.G.; Holzapfel, W.B. Effect of pressure on Raman spectra of solid chlorine. J. Phys. C Solid State Phys. 1983, 16, L1177–L1179. [Google Scholar] [CrossRef]
- Lockwood, D.J.; Bertrand, D.; Carrara, P.; Mischler, G.; Billerey, D.; Terrier, C. Raman spectrum of NiCl2. J. Phys. C Solid State Phys. 1979, 12, 3615–3620. [Google Scholar] [CrossRef]
- McGuire, M.A.; Yan, J.; Lampen-Kelley, P.; May, A.F.; Cooper, V.R.; Lindsay, L.; Puretzky, A.; Liang, L.; Kc, S.; Cakmak, E.; et al. High-temperature magnetostructural transition in van der Waals-layered α−MoCl3. Phys. Rev. Mater. 2017, 1, 064001. [Google Scholar] [CrossRef]
- Kazim, S.; Ali, M.; Palleschi, S.; D’Olimpio, G.; Mastrippolito, D.; Politano, A.; Gunnella, R.; Di Cicco, A.; Renzelli, M.; Moccia, G.; et al. Mechanical exfoliation and layer number identification of single crystal monoclinic CrCl3. Nanotechnology 2020, 31, 395706. [Google Scholar] [CrossRef]
- Somayazulu, M.; Gramsch, S.; Mao, H.-K.; Hemley, R. High Pressure-High Temperature Reactions in Xenon-Chlorine System. In Proceedings of the MRS Proceedings; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2006; Volume 987. [Google Scholar]
- Chaney, J.; Feigerle, C. Characterization of chlorinated chemical vapor deposited and natural (100) diamond. Surf. Sci. 1999, 425, 245–258. [Google Scholar] [CrossRef]
- Pravica, M.; Sneed, D.; Wang, Y.; Smith, Q.; Subrahmanyam, G. Carbon tetrachloride under extreme conditions. J. Chem. Phys. 2014, 140, 194503. [Google Scholar] [CrossRef] [PubMed]
- Brückner, D.C.R.; Haller, D.C.H.; Ellwanger, M.; Riedel, S. Polychloride Monoanions from [Cl3]− to [Cl9]−: A Raman Spectroscopic and Quantum Chemical Investigation. Chem. A Eur. J. 2012, 18, 5741–5747. [Google Scholar] [CrossRef] [PubMed]
- Brückner, R.; Haller, H.; Steinhauer, S.; Müller, C.; Riedel, S. A 2D Polychloride Network Held Together by Halogen-Halogen Interactions. Angew. Chem. Int. Ed. 2015, 54, 15579–15583. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Riedel, S. Recent Discoveries of Polyhalogen Anions—From Bromine to Fluorine. Z. Anorg. Allg. Chem. 2014, 640, 1281–1291. [Google Scholar] [CrossRef]
- Uhliar, M.; Tokár, K.; Kohulák, O.; Derzsi, M. Exploring crystal chemistry of binary silver chlorides with evolutionary algorithms and density functional theory. In Proceedings of the 2nd International Online Conference on Crystals, Online, 10–20 November 2020; MDPI: Basel, Switzerland, 2020. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzelak, A.; Gawraczyński, J.; Derzsi, M.; Struzhkin, V.; Somayazulu, M.; Grochala, W. Observation of a New Polyhalide Phase in Ag-Cl2 System at High Pressure. Crystals 2021, 11, 1565. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121565
Grzelak A, Gawraczyński J, Derzsi M, Struzhkin V, Somayazulu M, Grochala W. Observation of a New Polyhalide Phase in Ag-Cl2 System at High Pressure. Crystals. 2021; 11(12):1565. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121565
Chicago/Turabian StyleGrzelak, Adam, Jakub Gawraczyński, Mariana Derzsi, Viktor Struzhkin, Maddury Somayazulu, and Wojciech Grochala. 2021. "Observation of a New Polyhalide Phase in Ag-Cl2 System at High Pressure" Crystals 11, no. 12: 1565. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121565