Effects of Matrix Silicon Content on the Plasma Electrolytic Oxidation of Al-Si Alloys Using Different Power Modes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. PEO of Samples Using the PBC Oxidation Mode
3.2. PEO of Samples Using the PBV Oxidation Mode
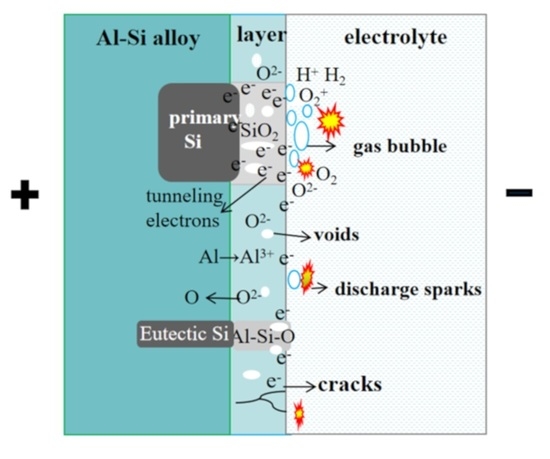
3.3. Mechanistic Analysis of the Impact of Si Grains on the PEO of Al-Si Alloys
4. Conclusions
- (1)
- The eutectic Si and primary Si grains had slow oxidation rates when using the pulsed bipolar voltage mode. It took a long time for the SiO2 growing on the large Si grains to mix with the surrounding Al2O3 to generate Al-Si-O compounds. The negative effect of porous SiO2 on the PEO process continued for a longer time in the Al alloy containing larger Si grains; therefore, the final layer thicknesses on different Al alloys decreased significantly with increasing Si content in the matrix;
- (2)
- The eutectic Si and primary Si grains were rapidly oxidized and converted to Al-Si-O compounds when using the pulsed bipolar current mode. The effect of Si or SiO2 on the layer growth process weakened after a short period of oxidation, such that the layer thicknesses on different Al alloys were very similar by the end of the pulsed bipolar voltage oxidation process;
- (3)
- The specific energy consumption of the layers decreased as the Si content increased, regardless of whether the pulsed bipolar current or pulsed bipolar voltage mode was applied.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.Q.; Wen, F.; Liu, C.; Li, W.R.; Zhou, Q.Y.; Zhu, W.C.; Zhang, Y.H.; Guan, R.G. The microstructure and property of Al-Si alloy improved by the Sc-microalloying and Y2O3 nano-particles. Sci. Technol. Adv. Mater. 2021, 22, 205–217. [Google Scholar] [CrossRef]
- Huang, J.M.; Zhao, H.D.; Chen, Z.M. Microstructure and properties of A356 alloy wheels fabricated by low-pressure die casting with local squeeze. J. Mater. Eng. Perform. 2019, 28, 2137–2146. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahat, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Lorusso, M.; Calignano, F.; Pavese, M.; Andreatta, F. Corrosion behavior of AlSi10Mg alloy produced by laser powder bed fusion under chloride exposures. Corros. Sci. 2019, 152, 101–108. [Google Scholar] [CrossRef]
- Rogov, A.B.; Lyu, H.; Matthews, A.; Yerokhin, A. AC plasma electrolytic oxidation of additively manufactured and cast AlSi12 alloys. Surf. Coat. Technol. 2020, 399, 126116. [Google Scholar] [CrossRef]
- Pezzato, L.; Dabalà, M.; Gross, S.; Brunelli, K. Effect of microstructure and porosity of AlSi10Mg alloy produced by selective laser melting on the corrosion properties of plasma electrolytic oxidation coatings. Surf. Coat. Technol. 2020, 404, 126477. [Google Scholar] [CrossRef]
- Zhu, B.W.; Zanella, C. Hardness and corrosion behaviour of anodised Al-Si produced by rheocasting. Mater. Des. 2019, 173, 107764. [Google Scholar] [CrossRef]
- Lien, H.H.; Mazumder, J.; Wang, J.; Misra, A. Microstructure evolution and high density of nanotwinned ultrafine Si in hypereutectic Al-Si alloy by laser surface remelting. Mater. Charact. 2020, 161, 110147. [Google Scholar] [CrossRef]
- Mora-Sanchez, H.; Olmo, R.; Rams, J.; Torres, B.; Mohedano, M.; Matykina, E.; Arrabal, R. Hard Anodizing and Plasma Electrolytic Oxidation of an Additively Manufactured Al-Si alloy. Surf. Coat. Technol. 2021, 420, 127339. [Google Scholar] [CrossRef]
- Wang, P.; Wang, P.; Yuwen, Q.Q.; Li, J.P. The differences in the formation mechanism of PEO and CPED composited ceramic coatings on Al-12Si alloy. J. Alloys Compd. 2019, 788, 61–66. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. Inst. Met. Finish. 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Shi, X.T.; Wang, Y.; Li, H.Y.; Zhang, S.F.; Zhao, R.F.; Li, G.Q.; Zhang, R.F.; Sheng, Y.; Cao, S.Y.; Zhao, Y.J.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Tran, Q.P.; Chin, T.S.; Kuo, Y.C.; Jin, C.X.; Trung, T.; Tuan, C.V.; Dang, D.Q. Diamond powder incorporated oxide layers formed on 6061 Al alloy by plasma electrolytic oxidation. J. Alloys Compd. 2018, 751, 289–298. [Google Scholar] [CrossRef]
- Haghighat-Shishavan, B.; Azari-Khosrowshahi, R.; Haghighat-Shishavan, S.; Nazarian-Samani, M.; Parvini-Ahmadi, N. Improving wear and corrosion properties of alumina coating on AA7075 aluminum by plasma electrolytic oxidation: Effects of graphite absorption. Appl. Surf. Sci. 2019, 481, 108–119. [Google Scholar] [CrossRef]
- Movahedi, N.; Habibolahzadeh, A. Effect of plasma electrolytic oxidation treatment on corrosion behavior of closed-cell Al-A356 alloy foam. Mater. Lett. 2016, 164, 558–561. [Google Scholar] [CrossRef]
- Yu, H.J.; Dong, Q.; Chen, Y.; Chen, C.Z. Influence of silicon on growth mechanism of micro-arc oxidation coating on cast Al-Si alloy. R. Soc. Open Sci. 2018, 5, 172428. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.B.; Shi, X.L.; Hua, M.; Li, Y.L. Preparation of anti-corrosion films by micro arc oxidation on an Al-Si alloy. Appl. Surf. Sci. 2007, 253, 6118–6124. [Google Scholar] [CrossRef]
- Zhu, M.H.; Cai, Z.B.; Lin, X.Z.; Ren, P.D.; Tan, J.; Zhou, Z.R. Fretting wear behaviour of ceramic coating prepared by micro-arc oxidation on Al-Si alloy. Wear 2007, 263, 472–480. [Google Scholar] [CrossRef]
- Krishna, L.R.; Purnima, A.S.; Wasekar, N.P.; Sundararajan, G. Kinetics and properties of micro arc oxidation coatings deposited on commercial Al alloys. Metall. Mater. Trans. A 2007, 38, 370–378. [Google Scholar] [CrossRef]
- Wang, L.; Nie, X. Silicon effects on formation of EPO oxide coatings on aluminum alloys. Thin Solid Films 2006, 494, 211–218. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.P.; Guo, Y.C.; Yang, Z.; Wang, J.L. Ceramic coating formation on high Si containing Al alloy by PEO process. Surf. Eng. 2016, 32, 428–434. [Google Scholar] [CrossRef]
- Xu, F.T.; Cai, Z.B.; Li, G. The mechanism of PEO process on Al-Si alloys with the bulk primary silicon. Appl. Surf. Sci. 2009, 255, 951–953. [Google Scholar] [CrossRef]
- Gulec, A.E.; Gencer, Y.; Tarakci, M. The characterization of oxide based ceramic coating synthesized on Al-Si binary alloys by microarc oxidation. Surf. Coat. Technol. 2015, 269, 100–107. [Google Scholar] [CrossRef]
- Mohedano, M.; Matykina, E.; Arrabal, R.; Mingo, B.; Zheludkevich, M.L. PEO of rheocast A356 Al alloy: Energy efficiency and corrosion properties. Surf. Interface Anal. 2016, 48, 953–959. [Google Scholar] [CrossRef]
- Li, K.; Li, W.F.; Zhang, G.G.; Zhu, W.; Zheng, F.H.; Zhang, D.Q.; Wang, M. Effects of Si phase refinement on the plasma electrolytic oxidation of eutectic Al-Si alloy. J. Alloys Compd. 2019, 790, 650–656. [Google Scholar] [CrossRef]
- Li, K.; Li, W.F.; Yi, A.H.; Zhu, W.; Liao, Z.M.; Chen, K.; Li, W.M. Tuning the Surface Characteristic of Al-Si Alloys and Its Impacts on the Formation of Micro Arc Oxidation Layers. Coatings 2021, 11, 453. [Google Scholar] [CrossRef]
- Hwang, I.J.; Hwang, D.Y.; Kim, Y.M.; Yoo, B.; Shin, D.H. Formation of uniform passive oxide layers on high Si content Al alloy by plasma electrolytic oxidation. J. Alloys Compd. 2010, 504, S527–S530. [Google Scholar] [CrossRef]
- Fernández-López, P.; Alves, S.A.; López-Ortega, A.; Jośe-Lombera, J.T.S.; Bayon, R. High performance tribological coatings on a secondary cast Al–Si alloy generated by Plasma Electrolytic Oxidation. Ceram. Int. 2021, 47, 31238–31250. [Google Scholar] [CrossRef]
- Mohedano, M.; Matykina, E.; Arrabal, R.; Mingo, B.; Pardo, A. PEO of pre-anodized Al–Si alloys: Corrosion properties and influence of sealings. Appl. Surf. Sci. 2015, 346, 57–67. [Google Scholar] [CrossRef]
- Fu, J.G.; Li, M.; Liu, G.S.; Ma, S.L.; Zhu, X.H.; Ma, C.S.; Cheng, D.; Yan, Z.J. Robust ceramic based self-lubricating coating on Al-Si alloys prepared via PEO and spin-coating methods. Wear 2020, 458–459, 203405. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Vijh, A.K. Sparking voltages and side reactions during anodization of valve metals in terms of electron tunneling. Corros. Sci. 1971, 11, 411–417. [Google Scholar] [CrossRef]
- Okorn-Schmidt, H.F. Characterization of silicon surface preparation processes for advanced gate dielectrics. IBM J. Res. Dev. 1999, 43, 351–365. [Google Scholar] [CrossRef]
- Adams, M. Ceramic Material Characteristics [EB/OL]. 2013. Available online: http://accuratus.com/materials.html (accessed on 14 May 2021).
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef] [Green Version]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhang, G.; Yi, A.; Zhu, W.; Liao, Z.; Chen, K.; Li, W.; Luo, Z. Effects of Matrix Silicon Content on the Plasma Electrolytic Oxidation of Al-Si Alloys Using Different Power Modes. Crystals 2022, 12, 123. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010123
Li K, Zhang G, Yi A, Zhu W, Liao Z, Chen K, Li W, Luo Z. Effects of Matrix Silicon Content on the Plasma Electrolytic Oxidation of Al-Si Alloys Using Different Power Modes. Crystals. 2022; 12(1):123. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010123
Chicago/Turabian StyleLi, Kang, Guoge Zhang, Aihua Yi, Wen Zhu, Zhongmiao Liao, Ken Chen, Wenfang Li, and Zhenyuan Luo. 2022. "Effects of Matrix Silicon Content on the Plasma Electrolytic Oxidation of Al-Si Alloys Using Different Power Modes" Crystals 12, no. 1: 123. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010123