Hierarchical Core/Shell Structured Ag@Ni(OH)2 Nanospheres as Binder-Free Electrodes for High Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
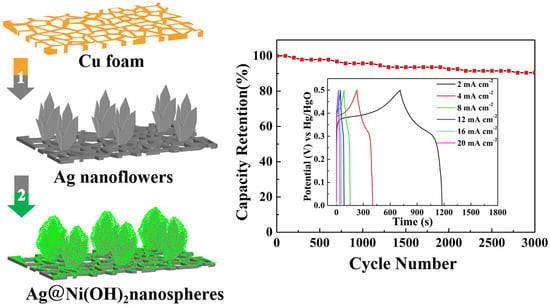
2.2. Synthesis of Hierarchical Ag@Ni(OH)2 Electrode
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Saha, S.; Samanta, P.; Murmu, N.C.; Kuila, T. A review on the heterostructure nanomaterials for supercapacitor application. J. Energy Storage 2018, 17, 181–202. [Google Scholar] [CrossRef]
- Guo, J.X.; Zhang, X.Q.; Sun, Y.F.; Zhang, X.H.; Tang, L.; Zhang, X. Double-shell CuS nanocages as advanced supercapacitor electrode materials. J. Power Sources 2017, 355, 31–35. [Google Scholar] [CrossRef]
- Dai, X.; Chen, D.; Fan, H.Q.; Zhong, Y.; Chang, L.; Shao, H.B.; Wang, J.M.; Zhang, J.Q.; Cao, C.N. Ni(OH)2/NiO/Ni composite nanotube arrays for high-performance supercapacitors. Electrochim. Acta 2015, 154, 128–135. [Google Scholar] [CrossRef]
- Liu, G.L.; Zhao, C.; Liu, T.Y.; He, D.; Suo, H. Facile route to achieve book-like tricobalt tetraoxide microstructures on copper foam for high performance supercapacitor. Mater. Lett. 2018, 220, 78–81. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.L.; Jia, S.Y.; Liu, X.C.; Wang, Y.H.; Xing, S.X. Facile route to achieve hierarchical hollow MnO2 nanostructures. Electrochim. Acta 2016, 203, 59–65. [Google Scholar] [CrossRef]
- Wang, T.; Pan, J.Q.; Achille, G.K.; Sun, Y.Z. A green dual complexation precipitation synthesis of hierarchical α-Ni(OH)2 microspheres and their electrochemical performance. Int. J. Hydrogen Energy 2017, 42, 19139–19147. [Google Scholar] [CrossRef]
- Wiston, B.R.; Ashok, M. Electrochemical performance of nickel hydroxide nanopetals for supercapacitor electrodes. Mater. Lett. 2019, 235, 76–79. [Google Scholar] [CrossRef]
- He, D.; Wang, G.D.; Liu, G.L.; Suo, H.; Zhao, C. Construction of leaf-like CuO-Cu2O nanocomposite on copper foam for high-performance supercapacitors. Dalton Trans. 2017, 46, 3318–3324. [Google Scholar] [CrossRef] [PubMed]
- Khatavkar, S.N.; Sartale, S.D. α-Fe2O3 thin film on stainless steel mesh: A flexible electrode for supercapacitor. Mater. Chem. Phys. 2019, 225, 284–291. [Google Scholar] [CrossRef]
- Lu, H.C.; Chen, J.Z.; Tian, Q.H. Wearable high-performance supercapacitors based on Ni-coated cotton textile with low-crystalline Ni-Al layered double hydroxide nanoparticles. J. Colloid Interface Sci. 2018, 513, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.Q.; Cheng, T.; Shen, X.Q.; Zhang, Y.Z.; Lai, W.Y.; Huang, W. Paper-based all-solid-state flexible asymmetric micro-supercapacitors fabricated by a simple pencil drawing methodology. Chin. Chem. Lett. 2018, 29, 587–591. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, L.; Yin, X.; Huang, T.; Gong, H. One step processed advanced interwoven architecture of Ni(OH)2 and Cu nanosheets with ultrahigh supercapacitor performance. J. Mater. Chem. A 2016, 4, 12144–12151. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Gokul Raja, T.S.; Purushothaman, S.; Kumar, M.V.; Sushmitha, I. Supercapacitive performances of MnO2 nanostructures grown on hierarchical Cu nano leaves via electrodeposition. Electrochim. Acta 2017, 227, 401–409. [Google Scholar] [CrossRef]
- Pawar, S.A.; Patil, D.S.; Shin, J.C. Hexagonal sheets of Co3O4 and Co3O4-Ag for high-performance electrochemical supercapacitors. J. Ind. Eng. Chem. 2017, 54, 162–173. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Shi, D.W.; Huang, T.J.; Huang, R.Z.; Gong, H. Light enhanced energy storage ability through hybrid plasmonic Ag nanowires decorated hydroxide “skin structure”. Nanoscale 2017, 9, 18430–18437. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, R.; Coskun, S.; Kalay, Y.E.; Unalan, H.E. Flexible, silver nanowire network nickel hydroxide core-shell electrodes for supercapacitors. J. Power Sources 2016, 328, 167–173. [Google Scholar] [CrossRef]
- Du, H.J.; Pan, Y.; Zhang, X.; Cao, F.Y.; Wan, T.; Du, H.W.; Joshi, R.; Chu, D.W. Silver nanowire/nickel hydroxide nanosheet composite for a transparent electrode and all-solid-state supercapacitor. Nanoscale Adv. 2019, 1, 140–146. [Google Scholar] [CrossRef]
- Wu, G.X.; Yang, S.J.; Liu, Q.M. Synthesis of silver nanostructures by simple redox under electrodeposited copper microcubes and the orient attachment growth of 2D silver. Appl. Surf. Sci. 2015, 357, 583–592. [Google Scholar] [CrossRef]
- Lo, I.H.; Wang, J.Y.; Huang, K.Y.; Huang, J.H.; Kang, W.P. Synthesis of Ni(OH)2 nanoflakes on ZnO nanowires by pulse electrodeposition for high-performance supercapacitors. J. Power Sources 2016, 308, 29–36. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.M.; Gerson, A.R.; Smart, R.C. The role of the Auger parameter in XPS studies of nickel metal, halides and oxides. Phys. Chem. Chem. Phys. 2012, 14, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.X.; Xie, S.L.; Liu, C.G. Flower-like Ni–Co hydroxides on Ni foam for high-performance supercapacitor applications. New J. Chem. 2018, 42, 4175–4181. [Google Scholar] [CrossRef]
- Sekhar, S.C.; Nagaraju, G.; Yu, J.S. Conductive silver nanowires-fenced carbon cloth fibers-supported layered double hydroxide nanosheets as a flexible and binder-free electrode for high-performance asymmetric supercapacitors. Nano Energy 2017, 36, 58–67. [Google Scholar] [CrossRef]
- Park, S.; Ming Tan, A.W.; Wang, J.X.; Lee, P.S. Coaxial Ag-base metal nanowire networks with high electrochemical stability for transparent and stretchable asymmetric supercapacitors. Nanoscale Horiz. 2017, 2, 199–204. [Google Scholar] [CrossRef]
- Cao, F.H.; Wang, Z.H.; Wang, Y.Z.; Yan, Y.M.; Liu, M.T.; Li, L.; Ao, G.H.; Chen, K.F.; Lv, Z. In situ fabrication of cellular architecture on silver metals using methane/oxygen gas mixture and its application for energy storage. Electrochim. Acta 2018, 280, 25–32. [Google Scholar] [CrossRef]
- Liu, P.P.; Liu, J.; Cheng, S.; Cai, W.Z.; Yu, F.Y.; Zhang, Y.P.; Wu, P.; Liu, M.L. A high-performance electrode for supercapacitors: Silver nanoparticles grown on a porous perovskite-type material La0.7Sr0.3CoO3-δ substrate. Chem. Eng. J. 2017, 328, 1–10. [Google Scholar] [CrossRef]
- Usman, M.; Pan, L.J.; Sohail, A.; Mahmood, Z.; Cui, R.X. Fabrication of 3D vertically aligned silver nanoplates on nickel foam-graphene substrate by a novel electrodeposition with sonication for efficient supercapacitors. Chem. Eng. J. 2017, 311, 359–366. [Google Scholar] [CrossRef]
- Shakir, I.; Ali, Z.; Bae, J.; Park, J.; Kang, D.J. Conformal coating of ultrathin Ni(OH)2 on ZnO nanowires grown on textile fiber for efficient flexible energy storage devices. RSC Adv. 2014, 4, 6324–6329. [Google Scholar] [CrossRef]
- Jia, X.-X.; Wu, X.; Liu, B.-D. Formation of ZnCo2O4@MnO2 core–shell electrode materials for hybrid supercapacitor. Dalton Trans. 2018, 47, 15506–15511. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.C.; Chu, Q.X.; Ren, X.B.; Ge, C.J.; Qusti, A.H.; Asiri, A.M.; Al-Youb, A.O.; Sun, X.P. Ni3S2 coated ZnO array for high-performance supercapacitors. J. Power Sources 2014, 245, 463–467. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Chu, X.; Yang, F.; Wang, H.; Yang, J.; Chi, Y.; Yang, X. Hierarchical Core/Shell Structured Ag@Ni(OH)2 Nanospheres as Binder-Free Electrodes for High Performance Supercapacitors. Crystals 2019, 9, 118. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020118
Lv S, Chu X, Yang F, Wang H, Yang J, Chi Y, Yang X. Hierarchical Core/Shell Structured Ag@Ni(OH)2 Nanospheres as Binder-Free Electrodes for High Performance Supercapacitors. Crystals. 2019; 9(2):118. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020118
Chicago/Turabian StyleLv, Sa, Xuefeng Chu, Fan Yang, Huan Wang, Jia Yang, Yaodan Chi, and Xiaotian Yang. 2019. "Hierarchical Core/Shell Structured Ag@Ni(OH)2 Nanospheres as Binder-Free Electrodes for High Performance Supercapacitors" Crystals 9, no. 2: 118. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020118