Polyetherimide Foams Filled with Low Content of Graphene Nanoplatelets Prepared by scCO2 Dissolution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Cellular Structure of the Foams
3.2. Thermal Analysis
3.3. Dynamic-Mechanical-Thermal Behavior
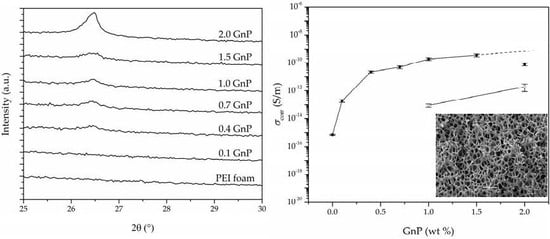
3.4. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKeen, L.W. Polyimides. In Permeability Properties of Plastics and Elastomers; William Andrew Publishing: Oxford, UK, 2012; pp. 107–120. ISBN 978-1-4377-3469-0. [Google Scholar]
- Gedler, G.; Antunes, M.; Velasco, J.I.; Ozisik, R. Enhanced electromagnetic interference shielding effectiveness of polycarbonate/graphene nanocomposites foamed via 1-step supercritical carbon dioxide process. Mater. Des. 2016, 90, 906–914. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.; Zhai, W.; Feng, W.; Shen, B.; Zhang, J.; Zheng, W. Ge Facile Preparation of Lightweight Microcellular Polyetherimide/Graphene Composite Foams for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2013, 5, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Graphene nanoplatelets-reinforced polyetherimide foams prepared by water vapor-induced phase separation. eXPRESS Polym. Lett. 2015, 9, 412–423. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J. Effects of Carbon Nanotubes/Graphene Nanoplatelets Hybrid Systems on the Structure and Properties of Polyetherimide-Based Foams. Polymers 2018, 10, 348. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Enhancing the electrical conductivity of polyetherimide-based foams by simultaneously increasing the porosity and graphene nanoplatelets dispersion. Polym. Compos. 2018. [Google Scholar] [CrossRef]
- Shen, B.; Zhai, W.; Tao, M.; Ling, J.; Zheng, W. Lightweight, Multifunctional Polyetherimide/Graphene@Fe3O4 Composite Foams for Shielding of Electromagnetic Pollution. ACS Appl. Mater. Interfaces 2013, 5, 11383–11391. [Google Scholar] [CrossRef]
- Nadella, K.; Kumar, V. Tensile and flexural properties of solid-state microcellular ABS panels. In Experimental Analysis of Nano and Engineering Materials and Structures; Springer: Berlin/Heidelberg, Germany, 2007; pp. 765–766. [Google Scholar]
- Martín-de León, J.; Bernardo, V.; Rodríguez-Pérez, M.Á. Low Density Nanocellular Polymers Based on PMMA Produced by Gas Dissolution Foaming: Fabrication and Cellular Structure Characterization. Polymers 2016, 8, 265. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Yu, C.-N.; Liang, Y.; Lin, G.-X.; Meng, C. Foaming Behavior and Microcellular Morphologies of Incompatible SAN/CPE Blends with Supercritical Carbon Dioxide as a Physical Blowing Agent. Polymers 2019, 11, 89. [Google Scholar] [CrossRef]
- Kumar, V.; VanderWel, M.; Weller, J.; Seeler, K.A. Experimental Characterization of the Tensile Behavior of Microcellular Polycarbonate Foams. J. Eng. Mater. Technol. 1994, 116, 439–445. [Google Scholar] [CrossRef]
- Shimbo, M.; Higashitani, I.; Miyano, Y. Mechanism of Strength Improvement of Foamed Plastics Having Fine Cell. J. Cell. Plast. 2007, 43, 157–167. [Google Scholar] [CrossRef]
- Miller, D.; Chatchaisucha, P.; Kumar, V. Microcellular and nanocellular solid-state polyetherimide (PEI) foams using sub-critical carbon dioxide I. Processing and structure. Polymer 2009, 50, 5576–5584. [Google Scholar] [CrossRef]
- Miller, D.; Kumar, V. Microcellular and nanocellular solid-state polyetherimide (PEI) foams using sub-critical carbon dioxide II. Tensile and impact properties. Polymer 2011, 52, 2910–2919. [Google Scholar] [CrossRef]
- Badamshina, E.; Estrin, Y.; Gafurova, M. Nanocomposites based on polyurethanes and carbon nanoparticles: Preparation, properties and application. J. Mater. Chem. A 2013, 1, 6509–6529. [Google Scholar] [CrossRef]
- Kostopoulos, V.; Vavouliotis, A.; Karapappas, P.; Tsotra, P.; Paipetis, A. Damage Monitoring of Carbon Fiber Reinforced Laminates Using Resistance Measurements. Improving Sensitivity Using Carbon Nanotube Doped Epoxy Matrix System. J. Intell. Mater. Syst. Struct. 2009, 20, 1025–1034. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Robinson, J.T.; Diankov, G.; Dai, H. Nanocrystal growth on graphene with various degrees of oxidation. J. Am. Chem. Soc. 2010, 132, 3270–3271. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Zhou, C.; Vaccaro, N.; Sundarram, S.S.; Li, W. Fabrication and characterization of polyetherimide nanofoams using supercritical CO2. J. Cell. Plast. 2012, 48, 239–255. [Google Scholar] [CrossRef]
- Gedler, G.; Antunes, M.; Velasco, J.I. Enhanced electrical conductivity in graphene-filled polycarbonate nanocomposites by microcellular foaming with sc-CO2. J. Adhes. Sci. Technol. 2016, 30, 1017–1029. [Google Scholar] [CrossRef]
- Ma, H.-L.; Zhang, H.-B.; Li, X.; Zhi, X.; Liao, Y.-F.; Yu, Z.-Z. The effect of surface chemistry of graphene on cellular structures and electrical properties of polycarbonate nanocomposite foams. Ind. Eng. Chem. Res. 2014, 53, 4697–4703. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Q.; Xing, Z.; Zhang, W.; Zhang, M.; Tan, H.; Wang, J.; Wu, G. Improving the Supercritical CO2 Foaming of Polypropylene by the Addition of Fluoroelastomer as a Nucleation Agent. Polymers 2019, 11, 226. [Google Scholar] [CrossRef]
- Ventura, H.; Sorrentino, L.; Laguna-Gutierrez, E.; Rodriguez-Perez, M.A.; Ardanuy, M. Gas Dissolution Foaming as a Novel Approach for the Production of Lightweight Biocomposites of PHB/Natural Fibre Fabrics. Polymers 2018, 10, 249. [Google Scholar] [CrossRef]
- Sims, G.L.A.; Khunniteekool, C. Cell-size measurement of polymeric foams. Cell. Polym. 1994, 13, 137–146. [Google Scholar]
- Van Krevelen, D.W.; Hoftyzer, P.J. Properties of Polymers: Their Estimation and Correlation with Chemical Structure; Elsevier: New York, NY, USA, 1976. [Google Scholar]
- Realinho, V.; Haurie, L.; Antunes, M.; Velasco, J.I. Thermal stability and fire behaviour of flame retardant high density rigid foams based on hydromagnesite-filled polypropylene composites. Compos. Part B Eng. 2014, 58, 553–558. [Google Scholar] [CrossRef]
- Yoon, O.J.; Jung, C.Y.; Sohn, I.Y.; Kim, H.J.; Hong, B.; Jhon, M.S.; Lee, N.-E. Nanocomposite nanofibers of poly(d,l-lactic-co-glycolic acid) and graphene oxide nanosheets. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1978–1984. [Google Scholar] [CrossRef]
- Ji, X.; Xu, Y.; Zhang, W.; Cui, L.; Liu, J. Review of functionalization, structure and properties of graphene/polymer composite fibers. Compos. Part A Appl. Sci. Manuf. 2016, 87, 29–45. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Z.; Zhang, J.; Cao, J.; Wang, S.; Tian, Q.; Gao, M.; Xu, Q. Fabrication of PVA/graphene oxide/TiO2 composite nanofibers through electrospinning and interface sol–gel reaction: Effect of graphene oxide on PVA nanofibers and growth of TiO2. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 318–325. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999; ISBN 131602542X. [Google Scholar]
- Kumar, V.; Nadella, K.V. Microcellular Foams. In Handbook of Polymer Foams; Eaves, D., Ed.; Rapa Technology Limited: Shawbury, UK, 2004; Volume 25, pp. 243–268. [Google Scholar]
- Zhou, J.; Yao, Z.; Chen, Y.; Wei, D.; Xu, T. Fabrication and mechanical properties of phenolic foam reinforced with graphene oxide. Polym. Compos. 2014, 35, 581–586. [Google Scholar]
- Yang, H.; Li, F.; Shan, C.; Han, D.; Zhang, Q.; Niu, L.; Ivaska, A. Covalent functionalization of chemically converted graphene sheets via silane and its reinforcement. J. Mater. Chem. 2009, 19, 4632–4638. [Google Scholar] [CrossRef]
- Chen, L.; Jin, H.; Xu, Z.; Shan, M.; Tian, X.; Yang, C.; Wang, Z.; Cheng, B. A design of gradient interphase reinforced by silanized graphene oxide and its effect on carbon fiber/epoxy interface. Mater. Chem. Phys. 2014, 145, 186–196. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Ji, J.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Primary and tertiary amines bifunctional graphene oxide for cooperative catalysis. Nanoscale 2013, 5, 6030–6033. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Su, S.; Kasner, M.L.; Shah, P.; Patel, K.; Madarang, C.J. Formation of highly stable dispersions of silane-functionalized reduced graphene oxide. Chem. Phys. Lett. 2010, 501, 68–74. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, J.; Song, M. Preparation and characterisation of covalent polymer functionalized graphene oxide. J. Mater. Chem. 2011, 21, 3455–3461. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I. Chemical functionalization of graphene with defects. Nano Lett. 2008, 8, 4373–4379. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2009, 114, 832–842. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Katsiotis, M.S.; Alhassan, S.M.; Liberatore, M.W.; Abdala, A.A. Effect of solvent on the uncatalyzed synthesis of aminosilane-functionalized graphene. RSC Adv. 2014, 4, 6830–6839. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Chiu, F.-C. A review on conduction mechanisms in dielectric films. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef]
- Antunes, M.; Mudarra, M.; Velasco, J.I. Broad-band electrical conductivity of carbon nanofibre-reinforced polypropylene foams. Carbon N. Y. 2011, 49, 708–717. [Google Scholar] [CrossRef]
- Sichel, E.K. Carbon Black-Polymer Composites: The Physics of Electrically Conducting Composites; Marcel Dekker Inc.: New York, NY, USA, 1982; Volume 3, ISBN 0824716736. [Google Scholar]
- Ryvkina, N.; Tchmutin, I.; Vilčáková, J.; Pelíšková, M.; Sáha, P. The deformation behavior of conductivity in composites where charge carrier transport is by tunneling: Theoretical modeling and experimental results. Synth. Met. 2005, 148, 141–146. [Google Scholar] [CrossRef]
- Hull, D.; Clyne, T.W. An Introduction to Composite Materials; Cambridge University Press: Cambridge, UK, 1996; ISBN 0521388554. [Google Scholar]
- Allaoui, A.; Hoa, S.V.; Pugh, M.D. The electronic transport properties and microstructure of carbon nanofiber/epoxy composites. Compos. Sci. Technol. 2008, 68, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Krenchel, H. Fibre Reinforcement; Alademisk forlag: Copenhagen, Denmark, 1964. [Google Scholar]
- Fisher, F.T.; Bradshaw, R.D.; Brinson, L.C. Fiber waviness in nanotube-reinforced polymer composites—I: Modulus predictions using effective nanotube properties. Compos. Sci. Technol. 2003, 63, 1689–1703. [Google Scholar] [CrossRef] [Green Version]









| Sample | GnP (wt %) | GnP (vol%) | Relative Density | Φ (μm) 1 | N0 (cells/cm3) |
|---|---|---|---|---|---|
| PEI | 0.0 | 0.00 | 0.44 | 14.0 (5.0) | 5.1 × 108 |
| 0.1 GnP | 0.1 | 0.03 | 0.48 | 11.7 (4.2) | 5.6 × 108 |
| 0.4 GnP | 0.4 | 0.11 | 0.49 | 13.6 (4.4) | 3.9 × 108 |
| 0.7 GnP | 0.7 | 0.17 | 0.42 | 5.4 (2.3) | 6.5 × 109 |
| 1.0 GnP | 1.0 | 0.27 | 0.46 | 9.5 (3.3) | 1.1 × 109 |
| 1.5 GnP | 1.5 | 0.35 | 0.40 | 10.0 (4.0) | 1.2 × 109 |
| 2.0 GnP | 2.0 | 0.57 | 0.49 | 7.5 (2.9) | 1.6 × 109 |
| Sample | Intensity (a.u.) | FWHM (°) |
|---|---|---|
| PEI | - | - |
| 0.1 GnP | - | - |
| 0.4 GnP | 350.5 | 0.23 |
| 0.7 GnP | 481.2 | 0.35 |
| 1.0 GnP | 505.4 | 0.30 |
| 1.5 GnP | 483.5 | 0.40 |
| 2.0 GnP | 1716.6 | 0.29 |
| Sample | Decomposition Temperature (°C) | CR (wt %) | LOI (%) | ||
|---|---|---|---|---|---|
| T5% weight loss | Tmax | T35% weight loss | |||
| PEI | 523.4 | 538.7 | 580.9 | 50.5 | 37.7 |
| 0.1 GnP | 516.8 | 537.3 | 578.3 | 50.8 | 37.8 |
| 0.4 GnP | 518.7 | 536.9 | 580.4 | 51.7 | 38.2 |
| 0.7 GnP | 516.2 | 536.5 | 579.3 | 51.5 | 38.1 |
| 1.0 GnP | 519.0 | 537.0 | 583.0 | 51.9 | 38.3 |
| 1.5 GnP | 517.4 | 536.6 | 582.9 | 52.4 | 38.5 |
| 2.0 GnP | 513.9 | 535.3 | 581.7 | 52.3 | 38.4 |
| Sample | Relative Density | E′ at 30 °C (MPa) | E′spec (MPa·cm3/g) | Glass Transition (°C) | |
|---|---|---|---|---|---|
| Max E″ | Max tanδ | ||||
| scCO2 foams | |||||
| PEI | 0.44 | 738.6 | 1295.8 | 212.0 | 220.9 |
| 0.1 GnP | 0.48 | 702.8 | 1171.3 | 212.1 | 224.4 |
| 0.4 GnP | 0.49 | 884.3 | 1426.3 | 212.5 | 221.4 |
| 0.7 GnP | 0.42 | 630.7 | 1168.0 | 212.6 | 224.0 |
| 1.0 GnP | 0.46 | 751.6 | 1273.9 | 212.8 | 218.2 |
| 1.5 GnP | 0.40 | 642.3 | 1235.2 | 213.0 | 224.2 |
| 2.0 GnP | 0.49 | 922.1 | 1463.7 | 210.9 | 226.5 |
| WVIPS foams 1 | |||||
| 1.0 GnP NS | 0.44 | 742.6 | 1335.6 | 218.0 | 225.0 |
| 2.0 GnP NS | 0.39 | 568.1 | 1147.7 | 218.4 | 226.7 |
| 1.0 GnP S | 0.26 | 370.4 | 1110.9 | 223.1 | 229.8 |
| 2.0 GnP S | 0.26 | 385.3 | 1170.5 | 223.3 | 228.6 |
| 2.0 CNT S | 0.44 | 442.9 | 776.5 | 221.5 | 227.1 |
| Sample | GnP (wt %) | GnP (vol%) | Porosity (%) | σ (S/m) | σcorr (S/m) 1 |
|---|---|---|---|---|---|
| PEI | 0.0 | 0.00 | 55.3 | 7.18 × 10−16 | 4.60 × 10−16 (9.92 × 10−17) |
| 0.1 GnP | 0.1 | 0.03 | 52.4 | 2.70 × 10−13 | 1.88 × 10−13 (4.03 × 10−14) |
| 0.4 GnP | 0.4 | 0.11 | 51.0 | 3.17 × 10−11 | 2.27 × 10−11 (4.10 × 10−12) |
| 0.7 GnP | 0.7 | 0.17 | 57.7 | 7.16 × 10−11 | 4.99 × 10−11 (1.25 × 10−11) |
| 1.0 GnP | 1.0 | 0.27 | 53.5 | 3.76 × 10−10 | 1.86 × 10−10 (4.00 × 10−11) |
| 1.5 GnP | 1.5 | 0.35 | 59.6 | 5.12 × 10−10 | 3.45 × 10−10 (8.67 × 10−11) |
| 2.0 GnP | 2.0 | 0.57 | 51.0 | 1.12 × 10−10 | 7.70 × 10−11 (1.53 × 10−11) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, H.; Antunes, M.; Velasco, J.I. Polyetherimide Foams Filled with Low Content of Graphene Nanoplatelets Prepared by scCO2 Dissolution. Polymers 2019, 11, 328. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020328
Abbasi H, Antunes M, Velasco JI. Polyetherimide Foams Filled with Low Content of Graphene Nanoplatelets Prepared by scCO2 Dissolution. Polymers. 2019; 11(2):328. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020328
Chicago/Turabian StyleAbbasi, Hooman, Marcelo Antunes, and José Ignacio Velasco. 2019. "Polyetherimide Foams Filled with Low Content of Graphene Nanoplatelets Prepared by scCO2 Dissolution" Polymers 11, no. 2: 328. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020328