Thermal Analysis and Crystal Structure of Poly(Acrylonitrile-Co-Itaconic Acid) Copolymers Synthesized in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PAN Homopolymer and PAN-IA Copolymers
2.3. Characterization
3. Results and Discussion
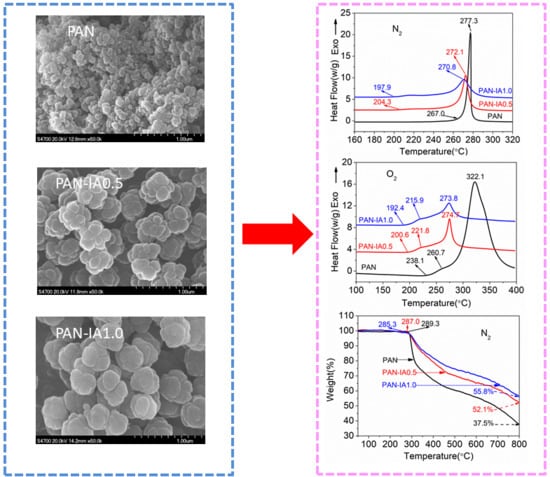
3.1. Morphology of the PAN Polymer
3.2. FTIR Analysis
3.3. XRD Analysis
3.4. Thermal Properties
3.5. Thermal Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, J.; Zhou, T.; Liu, X.; Yuan, Q.; Zhang, A. New understanding on the reaction pathways of the polyacrylonitrile copolymer fiber peroxidation: Online tracking by two-dimensional correlation FTIR spectroscopy. RSC Adv. 2016, 6, 4397–4409. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Sedghi, A.; Eslami, F.R. Effect of thermal characteristics of commercial and special polyacrylonitrile fibres on the fabrication of carbon fibers. Mater. Sci. Tech. 2006, 22, 1235–1239. [Google Scholar] [CrossRef]
- Newcomb, B.A. Processing, structure, and properties of carbon fibers. Compos. Part A Appl. S 2016, 91, 262–282. [Google Scholar] [CrossRef]
- Nunna, S.; Naebe, M.; Hameed, B.L.; Fox, B.L.; Creighton, C. Evolution of radial heterogeneity in polyacrylonitrile fibres during thermal stabilization: An overview. Polym. Degrad. Stabil. 2017, 136, 20–30. [Google Scholar] [CrossRef]
- Liu, S.; Han, K.; Chen, L.; Zheng, Y.; Yu, M. Influence of air circulation on the structure and properties of melt-spun PAN precursor fibers during thermal oxidation. RSC Adv. 2015, 5, 37669–37674. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Wu, G. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spoerl, J.M.; Buchmeiser, M.R. Chemlnform abstract: Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem. Int. Edit. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Bahl, O.P.; Mathur, R.B. Effect of load on the mechanical properties of carbon fibres from PAN precursor. Fibre Sci. Technol. 1979, 12, 31–37. [Google Scholar] [CrossRef]
- Faraji, S.; Yardim, M.F.; Can, D.S.; Sarac, A.S. Characterization of polyacrylonitrile, poly(acrylonitrile-co-vinyl acetate), and poly(acrylonitrile-co-itaconic acid) based activated carbon nanofibers. J. Appl. Polym. Sci. 2017, 134, 44381. [Google Scholar] [CrossRef]
- Ju, A.; Guang, S.; Xu, H. Effect of comonomer structure on the stabilization and spinnability of polyacrylonitrile copolymers. Carbon 2013, 54, 323–335. [Google Scholar] [CrossRef]
- Park, D.U.; Ryu, J.H.; Han, N.K.; Park, W.H.; Jeong, Y.G. Thermal analysis on the stabilization behavior of ternary copolymers based on acrylonitrile, methyl acrylate and itaconic acid. Fibers Polym. 2018, 19, 2439–2448. [Google Scholar] [CrossRef]
- Devasia, R.; Reghunadhan, N.C.P.; Sadhana, R.; Babu, N.S.; Ninan, K.N. Fourier transform infrared and wide-angle X-ray diffraction studies of the thermal cyclization reactions of high molar mass poly(acrylonitrile-co-itaconic acid). J. Appl. Polym. Sci. 2006, 100, 3055–3062. [Google Scholar] [CrossRef]
- Fu, Z.; Gui, Y.; Liu, S.; Wang, Z.; Liu, B.; Cao, C.; Zhang, H. Effects of an itaconic acid comonomer on the structural evolution and thermal behaviors of polyacrylonitrile used for polyacrylonitrile-based carbon fibers. J. Appl. Polym. Sci. 2014, 131, 5829–5836. [Google Scholar] [CrossRef]
- Liao, X.; Dulle, M.; Silva, J.M.S.; Wehrspohn, R.B.; Agarwal, S.; Forster, S.; Hou, H.; Smith, P.; Greiner, A. High strength in combination with high toughness in robust and sustainable polymeric material. Science 2019, 366, 1376–1379. [Google Scholar] [CrossRef]
- Fox, B. Making stronger carbon fiber precursors. Science 2019, 366, 1314–1315. [Google Scholar] [CrossRef]
- Minagawa, M.; Iwamatsu, J. Relation between the thermal behavior of polyacrylonitrile and polymerization factors. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 481–494. [Google Scholar] [CrossRef]
- Zeng, Z.P.; Zhang, Z.Z.; Xiao, R.; Lv, Y.G. Structures and properties of acrylonitrile (AN) and itaconic acid (IA) copolymers affected by polymerization reaction medium. Acta Polym. Sin. 2016, 9, 1229–1237. [Google Scholar]
- Bahrami, S.H.; Bajaj, P.; Sen, K. Thermal behavior of acrylonitrile carboxylic acid copolymers. J. Appl. Polym. Sci. 2003, 88, 685–698. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wang, Y.; Zhu, B. Aqueous deposited copolymerization of acrylonitrile and itaconic acid. J. Appl. Polym. Sci. 2009, 111, 3163–3169. [Google Scholar] [CrossRef]
- Morris, E.A.; Weisenberger, M.C.; Abdallah, M.G.; Vautard, F.V.; Grappe, H.; Ozcan, S.; Paulauskas, F.L.; Eberle, C.; Jackson, D.; Mecham, S.J.; et al. High performance carbon fibers from very high molecular weight polyacrylonitrile precursors. Carbon 2016, 101, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Liu, B.; Li, B.; Liu, Y.; Zhang, H. Comprehensive and quantitative study on the thermal oxidative stabilization reactions in poly(acrylonitrile-co-itaconic acid) copolymer. J. Appl. Polym. Sci. 2018, 135, 45934. [Google Scholar] [CrossRef]
- Arbab, S.; Zeinolebadi, A. Quantitative analysis of the effects of comonomers and heating conditions on the stabilization reactions of polyacrylonitrile fibers as carbon fiber precursors. Polym. Degrad. Stabil. 2017, 139, 107–116. [Google Scholar] [CrossRef]
- Hameed, N.; Sharp, J.; Nunna, S.; Creighton, C.; Magniez, K.; Jyotishkumar, P.; Salim, N.V.; Fox, B. Structural transformation of polyacrylonitrile fibers during stabilization and low temperature carbonization. Polym. Degrad. Stabil. 2016, 128, 39–45. [Google Scholar] [CrossRef]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Liu, J.; He, L.; Ma, S.; Liang, J.; Zhao, Y.; Fong, H. Effects of chemical composition and post-spinning stretching process on the morphological, structural, and thermo-chemical properties of electrospun polyacrylonitrile copolymer precursor nanofibers. Polymer 2015, 61, 20–28. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stabil. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Luo, Q.; Shi, Z.; Li, D.; Zhu, C.; Wang, M. DFT study on the ionic cyclization mechanism of copolymers of acrylonitrile-itaconic acid: Direct or autocatalytic? Chem. Phys. Lett. 2017, 687, 158–162. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.C.; Ahn, W.; Kim, C.Y. Reaction mechanisms of polyacrylonitrile on thermal treatment. Polym. Eng. Sci. 1993, 33, 1452–1457. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Liu, B.J.; Liu, Y.Y.; Li, B.; Zhang, H.X. Detailed cyclization pathways identification of polyacrylonitrile and poly(acrylonitrile-co-itaconic acid) by in situ FTIR and two-dimensional correlation analysis. Ind. Eng. Chem. Res. 2018, 57, 8348–8359. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Liu, B.J.; Deng, Y.J.; Ma, J.Y.; Cao, C.L.; Wang, J.; Ao, Y.H.; Zhang, H.X. The suitable itaconic acid content in polyacrylonitrile copolymers used for PAN-based carbon fibers. J. Appl. Polym. Sci. 2016, 133, 43919. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, C.P.R.; Sivadasan, P.; Katherine, B.K.; Ninan, K.N. Cyclization reaction in poly(acrylonitrile/itaconic acid) copolymer: An isothermal differential scanning calorimetry kinetic study. J. Appl. Polym. Sci. 2003, 88, 915–920. [Google Scholar] [CrossRef]
- Nguyenthai, N.U.; Hong, S.C. Structural evolution of poly(acrylonitrile-co-itaconic acid) during thermal oxidative stabilization for carbon materials. Macromolecules 2013, 46, 5882–5889. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. DSC study of stabilization reactions in poly(acrylonitrile-co-itaconic acid) with peak resolving method. J. Therm. Anal. Calorim. 2008, 94, 85–88. [Google Scholar] [CrossRef]
- Zeng, Z.P.; Shao, Z.C.; Xiao, R.; Lu, Y.G. Structure evolution mechanism of poly(acrylonitrile/itaconic acid/acrylamide) during thermal oxidative stabilization process. Chin. J. Polym. Sci. 2017, 35, 1020–1034. [Google Scholar] [CrossRef]
- Yu, M.J.; Wang, C.G.; Zhao, Y.Q.; Zhang, M.; Wang, W.Q. Thermal properties of acrylonitrile/itaconic acid polymers in oxidative and nonoxidative. J. Appl. Polym. Sci. 2010, 116, 1207–1212. [Google Scholar] [CrossRef]
- Shlyakhtin, A.V.; Lemenovskii, D.A.; Nifant’ev, I.E. Thermal behavior of the copolymers of acrylonitrile with methyl acrylate and itaconic acid or its derivatives. Mendeleev Commun. 2013, 23, 277–278. [Google Scholar] [CrossRef]
- Nguyenthai, N.U.; Hong, S.C. Controlled architectures of poly(acrylonitrile-co-itaconic acid) for efficient structural transformation into carbon materials. Carbon 2014, 69, 571–581. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Yang, J.; Ji, M.; Yu, J.; Wang, M.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Preparation, stabilization and carbonization of a novel polyacrylonitrile based carbon fiber precursor. Polymers 2019, 11, 1150. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Quan, L.; Xu, L. Effects of amino-functionalized carbon nanotubes on the crystal structure and thermal properties of polyacrylonitrile homopolymer microspheres. Polymers 2017, 9, 332. [Google Scholar] [CrossRef] [Green Version]
- Shibo, H.; Desimone, J.M. Dispersion polymerization of acrylonitrile in supercritical carbon dioxide. Macromolecules 2000, 33, 1565–1569. [Google Scholar]
- Zhang, Z.; Jiang, W.; Wang, Y.; Wu, Y.; Hou, X. Synthesis of monodispersed polyacrylonitrile microspheres by dispersion polymerization. J. Appl. Polym. Sci. 2012, 125, 4142–4148. [Google Scholar] [CrossRef]
- Simitzis, J.C.; Georgiou, P.C. Functional group changes of polyacrylonitrile fibres during their oxidative, carbonization and electrochemical treatment. Mater. Sci. 2015, 50, 4547–4564. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, L.; Shi, F.; Li, C.; Liu, H.; Xu, L. Rheological behavior of amino-functionalized multi-walled carbon nanotube/polyacrylonitrile concentrated solutions and crystal structure of composite fibers. Polymers 2018, 10, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Q.; Wang, X.; Wang, X.; Huang, J.; Huang, X.; Chen, Y. Simultaneous DSC/TG analysis on the thermal behavior of PAN polymers prepared by aqueous free-radical polymerization. Polym. Degrad. Stabil. 2016, 130, 320–327. [Google Scholar] [CrossRef]
- Yu, M.J.; Bai, Y.J.; Wang, C.G.; Xu, Y.; Guo, P.A. A new method for the evaluation of stabilization index of polyacrylonitrile fibers. Mater. Lett. 2007, 61, 2292–2294. [Google Scholar] [CrossRef]
- Chen, H.; Pan, Y.; Hou, S.; Shao, Z.; Hong, Y.; Ju, A. Poly(acrylonitrile-co-2-methylenesuccinamic acid) as a potential carbon fiber precursor: Preparation and stabilization. RSC Adv. 2017, 7, 54142. [Google Scholar] [CrossRef] [Green Version]









| Samples | The Value of Absorbance | A1735/A2243 | |
|---|---|---|---|
| 2243 cm−1 | 1735 cm−1 | ||
| Polyacrylonitrile (PAN)-IA0.5 | 0.2945 | 0.0468 | 0.1589 |
| PAN-IA1.0 | 0.5450 | 0.1713 | 0.3143 |
| Samples | Degree of Crystallinity (%) | Crystal Size (Å) |
|---|---|---|
| PAN | 35.05 | 53.40 |
| PAN-IA0.5 | 28.98 | 50.46 |
| PAN-IA1.0 | 27.17 | 49.17 |
| Samples-N2 | Ti (°C) | Tf (°C) | Tm (°C) | ΔH (J g−1) | ΔT (°C) | ΔH/ΔT (J g−1 °C−1) |
|---|---|---|---|---|---|---|
| PAN | 267.0 | 281.5 | 277.3 | 567.1 | 14.5 | 39.1 |
| PAN-IA0.5 | 204.3 | 287.6 | 272.1 | 526.7 | 83.3 | 6.32 |
| PAN-IA1.0 | 197.9 | 295.6 | 270.8 | 534.1 | 97.7 | 5.47 |
| Samples-O2 | Ti (°C) | Tm1 (°C) | Tm2 (°C) | Tf (°C) | ΔH (J g−1) | ΔT (°C) | ΔH/ΔT (J g−1 °C−1) |
|---|---|---|---|---|---|---|---|
| PAN | 238.1 | 260.7 | 322.1 | 375.2 | 4738 | 137.1 | 34.6 |
| PAN-IA0.5 | 200.6 | 221.8 | 274.7 | 368.2 | 1112 | 167.6 | 6.64 |
| PAN-IA1.0 | 192.4 | 215.9 | 273.8 | 364.6 | 1090 | 172.2 | 6.33 |
| Samples | Initial Decomposition Temperature (°C) | Residual Weight at 285 °C (%) | Residual Weight at 350 °C (%) | Residual Weight at 500 °C (%) | Residual Weight at 800 °C (%) |
|---|---|---|---|---|---|
| PAN | 289.3 | 99.1 | 73.3 | 60.4 | 37.5 |
| PAN-IA0.5 | 287.0 | 98.7 | 85.4 | 70.2 | 52.1 |
| PAN-IA1.0 | 285.3 | 98.2 | 87.2 | 74.7 | 55.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Quan, L.; Gao, A.; Tong, Y.; Shi, F.; Xu, L. Thermal Analysis and Crystal Structure of Poly(Acrylonitrile-Co-Itaconic Acid) Copolymers Synthesized in Water. Polymers 2020, 12, 221. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010221
Zhang H, Quan L, Gao A, Tong Y, Shi F, Xu L. Thermal Analysis and Crystal Structure of Poly(Acrylonitrile-Co-Itaconic Acid) Copolymers Synthesized in Water. Polymers. 2020; 12(1):221. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010221
Chicago/Turabian StyleZhang, Hailong, Ling Quan, Aijun Gao, Yuping Tong, Fengjun Shi, and Lianghua Xu. 2020. "Thermal Analysis and Crystal Structure of Poly(Acrylonitrile-Co-Itaconic Acid) Copolymers Synthesized in Water" Polymers 12, no. 1: 221. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010221