White Graphene-Cobalt Oxide Hybrid Filler Reinforced Polystyrene Nanofibers for Selective Oil Absorption
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Synthesis of Cobalt Oxide
2.3. Synthesis of hBN/Co3O4 Hybrid Nanofiller
2.4. Synthesis of PS Nanocomposites by Electrospinning
2.5. Characterization Techniques
3. Results and Discussion
3.1. Morphology and Structural Analysis of the Synthesized Nanomaterials
3.2. Morphology and Structural Analysis of the PS Nanocomposite Fibers
3.3. Mechanical Properties of the PS Nanocomposite Fibers
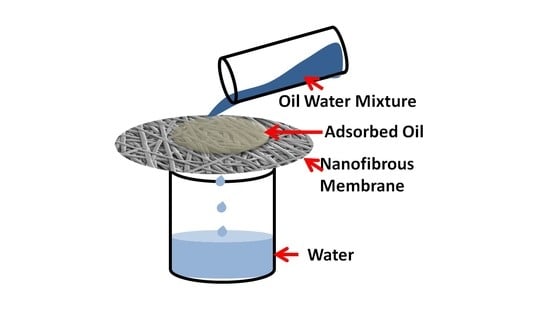
3.4. Surface Wettability of the Nanocomposite Fibers by Oil and Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Karki, H.P.; Kafle, L.; Ojha, D.P.; Song, J.H.; Kim, H.J. Cellulose/polyacrylonitrile electrospun composite fiber for effective separation of the surfactant-free oil-in-water mixture under a versatile condition. Sep. Purif. Technol. 2019, 210, 913–919. [Google Scholar] [CrossRef]
- Lee, M.W.; An, S.; Latthe, S.S.; Lee, C.; Hong, S.; Yoon, S.S. Electrospun polystyrene nanofiber membrane with superhydrophobicity and superoleophilicity for selective separation of water and low viscous oil. ACS Appl. Mater. Interfaces 2013, 5, 10597–10604. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Boots, B.; Blockley, D.J.; Rocha, C.; Thompson, R. Impacts of discarded plastic bags on marine assemblages and ecosystem functioning. Environ. Sci. Technol. 2015, 49, 5380–5389. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, J.L. The gulf oil spill. Environ. Sci. Technol. 2010, 44, 4833. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2016, 19, 403–414. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An intelligent superwetting PVDF membrane showing switchable transport performance for oil/water separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, N.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Electrospun porous structure fibrous film with high oil adsorption capacity. ACS Appl. Mater. Interfaces 2012, 4, 3207–3212. [Google Scholar] [CrossRef]
- Tai, M.H.; Gao, P.; Tan, B.Y.L.; Sun, D.D.; Leckie, J.O. Highly efficient and flexible electrospun carbon–silica nanofibrous membrane for ultrafast gravity-driven oil-water separation. ACS Appl. Mater. Interfaces 2014, 6, 9393–9401. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, J.; Feng, L.; Jiang, L.; Tang, W.; Luo, X.; Han, C.C. Facile creation of a super-amphiphobic coating surface with bionic microstructure. Adv. Mater. 2004, 16, 302–305. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Reviews 2010, 39, 3240–3255. [Google Scholar] [CrossRef]
- Li, J.; Shi, L.; Chen, Y.; Zhang, Y.; Guo, Z.; Su, B.L.; Liu, W. Stable superhydrophobic coatings from thiol-ligand nanocrystals and their application in oil/water separation. J. Mater. Chem. 2012, 22, 9774–9781. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Zheng, H.; Bai, R. A facile approach for the fabrication of highly stable superhydrophobic cotton fabric with multi-walled carbon nanotubes-azide polymer composites. Langmuir 2010, 26, 7529–7534. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, A.; Li, J.; Xu, J.; Han, C.C. Studies on the controlled morphology and wettability of polystyrene surfaces by electrospinning or electrospraying. Polymer 2006, 47, 7095–7102. [Google Scholar] [CrossRef]
- Kang, M.; Jung, R.; Kim, H.S.; Jin, H.J. Preparation of superhydrophobic polystyrene membranes by electrospinning. Coll. Surf. A Physicochem. Eng. Aspects 2008, 313, 411–414. [Google Scholar] [CrossRef]
- Salehirad, M.; Nikje, M.M.A. Synthesis and characterization of exfoliated polystyrene grafted hexagonal boron nitride nanosheets and their potential application in heat transfer nanofluids. Iran. Poly. J. 2017, 26, 467–480. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, W.; Wang, T.; Lu, Y. Highly hydrophobic, compressible, and magnetic polystyrene/Fe3O4/graphene aerogel composite for oil-water separation. Ind. Eng. Chem. Res. 2015, 54, 5460–5467. [Google Scholar] [CrossRef]
- Thangamani, J.G.; Deshmukh, K.; Sadasivuni, K.K.; Ponnamma, D.; Goutham, S.; Rao, K.V.; Pasha, S.K. White graphene reinforced polypyrrole and poly (vinyl alcohol) blend nanocomposites as chemiresistive sensors for room temperature detection of liquid petroleum gases. Microchim. Acta 2017, 184, 3977–3987. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Terao, T.; Tang, C.; Kuwahara, H.; Golberg, D. Towards thermoconductive, electrically insulating polymeric composites with boron nitride nanotubes as fillers. Adv. Funct. Mater. 2009, 19, 1857–1862. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Liu, Y.; Craig, V.; Li, L.H.; Chen, Y. Superhydrophobic and superoleophilic boron nitride nanotube-coated stainless steel meshes for oil and water separation. Adv. Mater. Interfaces 2014, 1, 1300002–1300007. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Narayanan, T.N.; Hashim, D.P.; Sakhavand, N.; Shahsavari, R.; Vajtai, R.; Ajayan, P.M. Hexagonal boron nitride and graphite oxide reinforced multifunctional porous cement composites. Adv. Funct. Mater. 2013, 23, 5624–5630. [Google Scholar] [CrossRef]
- Lee, C.H.; Drelich, J.; Yap, Y.K. Superhydrophobicity of boron nitride nanotubes grown on silicon substrates. Langmuir 2009, 25, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, J.; Wang, G.; Liang, W.; Zhang, Y.; Shi, L.; Guo, Z.; Liu, W. Methodology for robust superhydrophobic fabrics and sponges from in situ growth of transition metal/metal oxide nanocrystals with thiol modification and their applications in oil/water separation. ACS Appl. Mat. Interfaces 2013, 5, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Cruz-Yusta, M.; Gasparotto, A.; Maccato, C.; Morales, J.; Pozza, A.; Sada, C.; Sánchez, L.; Tondello, E. Cobalt oxide nanomaterials by vapor-phase synthesis for fast and reversible lithium storage. J. Phys. Chem. C 2010, 114, 10054–10060. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Zhang, J.; Xiang, J.Y.; Wang, X.L.; Zhao, X.B. Cobalt oxide ordered bowl-like array films prepared by electrodeposition through monolayer polystyrene sphere template and electrochromic properties. ACS Appl. Mat. Interfaces 2009, 2, 186–192. [Google Scholar] [CrossRef]
- Ponnamma, D.; Goutham, S.; Sadasivuni, K.K.; Rao, K.V.; Cabibihan, J.J.; Al-Maadeed, M.A.A. Controlling the sensing performance of rGO filled PVDF nanocomposite with the addition of secondary nanofillers. Synthetic Metals 2018, 243, 34–43. [Google Scholar] [CrossRef]
- Chamakh, M.M.; Ponnamma, D.; Al-Maadeed, M.A.A. Vapor sensing performances of PVDF nanocomposites containing titanium dioxide nanotubes decorated multi-walled carbon nanotubes. J. Mater. Sci. Mater. Electron. 2018, 29, 4402–4412. [Google Scholar] [CrossRef]
- Ponnamma, D.; Erturk, A.; Parangusan, H.; Deshmukh, K.; Ahamed, M.B.; Al-Maadeed, M.A. Stretchable quaternary phasic PVDF-HFP nanocomposite films containing graphene-titania-SrTiO3 for mechanical energy harvesting. Emerg. Mater. 2018, 1, 55–65. [Google Scholar] [CrossRef]
- Ponnamma, D.; Al-Maadeed, M.A.A. 3D architectures of titania nanotubes and graphene with efficient nanosynergy for supercapacitors. Mater. Des. 2017, 117, 203–212. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Pasha, S.K.; Sadasivuni, K.K.; Ponnamma, D.; Chidambaram, K. Synergistic effect of vanadium pentoxide and graphene oxide in polyvinyl alcohol for energy storage application. Eur. Polym. J. 2016, 76, 14–27. [Google Scholar] [CrossRef]
- Ni, Y.; Ge, X.; Zhang, Z.; Liu, H.; Zhu, Z.; Ye, Q. A simple reduction-oxidation route to prepare Co3O4 nanocrystals. Mater. Res. Bull. 2001, 36, 2383–2387. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2009, 114, 111–119. [Google Scholar] [CrossRef]
- Figlarz, M.; Guenot, J.; Tournemolle, J.N. Oxidation of cobalt (II) hydroxide to oxide hydroxide: Solids evolution during reaction. J. Mater. Sci. 1974, 9, 772–776. [Google Scholar] [CrossRef]
- Fischer, N.; Van Steen, E.; Claeys, M. Preparation of supported nano-sized cobalt oxide and fcc cobalt crystallites. Catal. Today 2011, 171, 174–179. [Google Scholar] [CrossRef]
- Hou, Y.; Wen, Z.; Cui, S.; Ci, S.; Mao, S.; Chen, J. Hybrid electrocatalysis: An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting (Adv. Funct. Mater. 6/2015). Adv. Funct. Mater. 2015, 25, 871. [Google Scholar] [CrossRef] [Green Version]
- Ai, L.H.; Jiang, J. Rapid synthesis of nanocrystalline Co3O4 by a microwave-assisted combustion method. Powder Technol. 2009, 195, 11–14. [Google Scholar] [CrossRef]
- Askarinejad, A.; Morsali, A. Direct ultrasonic-assisted synthesis of sphere-like nanocrystals of spinel Co3O4 and Mn3O4. Ultrasonics Sonochem. 2009, 16, 124–131. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, F.; Li, D.; Zhang, X. Large-scale Co3O4 nanoparticles growing on nickel sheets via a one-step strategy and their ultra-highly reversible redox reaction toward supercapacitors. J. Mater. Chem. 2011, 21, 18183–18185. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Raja, A.; Devarajan, P.A. Structural elucidation and spectral characterizations of Co3O4 nanoflakes. Spectrochim. Acta A Mol. Biomol. Spectr. 2013, 114, 586–591. [Google Scholar] [CrossRef]
- Parangusan, H.; Ponnamma, D.; AlMaadeed, M.A. Investigation on the Effect of γ- irradiation on the dielectric and piezoelectric properties of stretchable PVDF/Fe-ZnO nanocomposites for self-powering devices. Soft Matt. 2018, 14, 8803–8813. [Google Scholar] [CrossRef]
- Parangusan, H.; Ponnamma, D.; Adham, S.; Hassan, M.K.; Karim, A.; AlMaadeed, M.A. Designing carbon nanotube-based oil absorbing membranes from gamma irradiated and electrospun polystyrene nanocomposites. Materials 2019, 12, 709. [Google Scholar] [CrossRef] [Green Version]
- Bergshoef, M.M.; Vancso, G.J. Transparent nanocomposites with ultrathin, electrospun nylon-4, 6 fiber reinforcement. Adv. Mater. 1999, 11, 1362–1365. [Google Scholar] [CrossRef]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Cheng, Y.; Lin, Q.; Yang, B. Facile approach in fabricating superhydrophobic and superoleophilic surface for water and oil mixture separation. ACS Appl. Mater. Interfaces 2009, 1, 2613–2617. [Google Scholar] [CrossRef] [PubMed]









| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | |
|---|---|---|---|---|
| Non irradiated | PS | 28.54 ± 1.1 | 72.50 ± 6.44 | 18.12 ± 1.22 |
| PS/Co-O | 30.47 ± 2.3 | 76.33 ± 2.21 | 17.24 ± 1.10 | |
| PS/hBN | 35.44 ± 2.5 | 77.65 ± 4.05 | 17.39 ± 0.98 | |
| PS/hBCo-O | 48.24 ± 2.6 | 98.15 ± 4.79 | 10.23 ± 0.77 | |
| Irradiated | PS | 30.87 ± 1.3 | 73.30 ± 9.01 | 19.90 ± 1.01 |
| PS/Co-O | 31.05 ± 2.2 | 77.88 ± 8.75 | 17.90 ± 1. 12 | |
| PS/hBN | 38.25 ± 1.7 | 82.03 ± 2.45 | 16.35 ± 2.25 | |
| PS/hBCo-O | 52.54 ± 2.1 | 110.35 ± 4.55 | 9.50 ± 2.15 | |
| Sample | Weight before Oil Absorption | Weight after Oil Absorption | Increase in Weight | |
|---|---|---|---|---|
| Non irradiated | PS | 0.1208 | 0.8413 | 0.7205 |
| PS/Co-O | 0.1263 | 1.1762 | 1.0499 | |
| PS/hBN | 0.0921 | 1.273 | 1.1809 | |
| PS/hBCo-O | 0.1035 | 1.3140 | 1.2105 | |
| Irradiated | PS | 0.0526 | 0.7761 | 0.7235 |
| PS/Co-O | 0.1193 | 1.7546 | 1.6353 | |
| PS/hBN | 0.1244 | 1.4430 | 1.3186 | |
| PS/hBCo-O | 0.3838 | 2.592 | 2.2082 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnamma, D.; S Nair, S.; Parangusan, H.; K. Hassan, M.; Adham, S.; Karim, A.; Al Ali Al-Maadeed, M. White Graphene-Cobalt Oxide Hybrid Filler Reinforced Polystyrene Nanofibers for Selective Oil Absorption. Polymers 2020, 12, 4. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010004
Ponnamma D, S Nair S, Parangusan H, K. Hassan M, Adham S, Karim A, Al Ali Al-Maadeed M. White Graphene-Cobalt Oxide Hybrid Filler Reinforced Polystyrene Nanofibers for Selective Oil Absorption. Polymers. 2020; 12(1):4. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010004
Chicago/Turabian StylePonnamma, Deepalekshmi, Sabari S Nair, Hemalatha Parangusan, Mohammad K. Hassan, Samer Adham, Alamgir Karim, and Mariam Al Ali Al-Maadeed. 2020. "White Graphene-Cobalt Oxide Hybrid Filler Reinforced Polystyrene Nanofibers for Selective Oil Absorption" Polymers 12, no. 1: 4. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010004