Progress in the Development of Chitosan Based Insulin Delivery Systems: A Systematic Literature Review
Abstract
:1. Introduction
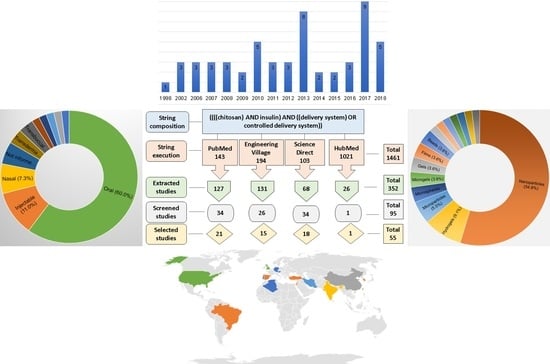
2. Materials and Methods
2.1. Search Strategy
2.2. Research Questions
2.3. Primary Study Selection
2.4. Supplementary Material
3. Results and Discussion
3.1. Overview of the Articles
3.2. Research Questions (RQ)
3.2.1. RQ1. At What Stage of Development Is the Chitosan/Insulin Delivery System?
3.2.2. RQ2. What Are the Release Systems Developed?
Nanoparticles
Gel (Hydrogel and Microgel)
Microparticles (Microspheres and Beads)
Films
3.2.3. RQ3. What Are the Main Alternative Ways of Administration Suggested?
3.2.4. RQ4. What Is the Total Amount of Insulin Loaded and the Encapsulation Efficiency of the Release System?
3.2.5. RQ5. For How Long Did the System Release the Insulin?
4. Risk of Biases
4.1. Primary Study Identification
4.2. Quality of Studies and Data Extraction Consistency
4.3. Data Synthesis and Results Reporting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes. 2017. Available online: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf (accessed on 12 December 2017).
- International Diabetes Federation. Diabetes Atlas. 2017. Available online: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html (accessed on 12 December 2017).
- Woo, V.C. New insulins and new aspects in insulin delivery. Can. J. Diabetes 2015, 39, 335–343. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diabetes Care: Standards of Medical Care in Diabetes—2017. 2017. Available online: http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf (accessed on 12 December 2017).
- Grunberger, G. The need for better insulin therapy. Diabetes Obes. Metab. 2013, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khan Ghilzai, N.M. New developments in insulin delivery. Drug Dev. Ind. Pharm. 2003, 29, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Mousavi, S.N. Synthesis of a novel structure for the oral delivery of insulin and the study of its effect on diabetic rats. Life Sci. 2017, 186, 43–49. [Google Scholar] [CrossRef]
- Barbari, G.R.; Dorkoosh, F.A.; Amini, M.; Sharifzadeh, M.; Atyabi, F.; Balalaie, S.; Tehrani, N.R.; Tehrani, M.R. A novel nanoemulsion-based method to produce ultrasmall, water-dispersible nanoparticles from chitosan, surface modified with cell-penetrating peptide for oral delivery of proteins and peptides. Int. J. Nanomed. 2017, 12, 3471. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Zhou, X.; Wu, K.; Long, R.; Wang, S.; Huang, H.; Xia, Y.; Liu, Y. The influence of spatial distribution on add-on therapy of designed Ca-Alg/CS MEMs system. J. Biomater. Sci. Polym. Ed. 2018, 29, 1319–1330. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.-J.; Zhao, H.-y.; Zheng, J.-M.; Xu, H.; Wei, G.; Hao, J.-S. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- Ubaidulla, U.; Khar, R.K.; Ahmed, F.J.; Panda, A.K. Development and in-vivo evaluation of insulin-loaded chitosan phthalate microspheres for oral delivery. J. Pharm. Pharmacol. 2007, 59, 1345–1351. [Google Scholar] [CrossRef]
- Hecq, J.; Siepmann, F.; Siepmann, J.; Amighi, K.; Goole, J. Development and evaluation of chitosan and chitosan derivative nanoparticles containing insulin for oral administration. Drug Dev. Ind. Pharm. 2015, 41, 2037–2044. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D. Development and characterization of new insulin containing polysaccharide nanoparticles. Colloids Surf. B Biointerfaces 2006, 53, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Sonaje, K.; Lin, K.-J.; Wey, S.-P.; Lin, C.-K.; Yeh, T.-H.; Nguyen, H.-N.; Hsu, C.-W.; Yen, T.-C.; Juang, J.-H.; Sung, H.-W. Biodistribution, pharmacodynamics and pharmacokinetics of insulin analogues in a rat model: Oral delivery using pH-responsive nanoparticles vs. subcutaneous injection. Biomaterials 2010, 31, 6849–6858. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Jiang, T.; Di, J.; Tai, W.; Gu, Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014, 43, 3595–3629. [Google Scholar] [CrossRef]
- Lancina III, M.G.; Shankar, R.K.; Yang, H. Chitosan nanofibers for transbuccal insulin delivery. J. Biomed. Mater. Res. Part A 2017, 105, 1252–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, F.; He, C.; He, M.; Tang, C.; Yin, L.; Qian, F.; Yin, C. Preparation and evaluation of chitosan-ethylenediaminetetraacetic acid hydrogel films for the mucoadhesive transbuccal delivery of insulin. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 89, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. An integrated buccal delivery system combining chitosan films impregnated with peptide loaded PEG-b-PLA nanoparticles. Colloids Surf. B Biointerfaces 2013, 112, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.; Hinchcliffe, M.; Watts, P.; Castile, J.; Jabbal-Gill, I.; Nankervis, R.; Smith, A.; Illum, L. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm. Res. 2002, 19, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Gupta, P.; Khanna, A.; Sharma, R.; Chandrabanshi, H.; Gupta, N.; Patil, U.; Yadav, S. Development and characterization of in situ gel system for nasal insulin delivery. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 188–193. [Google Scholar]
- Zhang, X.; Zhang, H.; Wu, Z.; Wang, Z.; Niu, H.; Li, C. Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 68, 526–534. [Google Scholar] [CrossRef]
- Finotelli, P.V.; Da Silva, D.; Sola-Penna, M.; Rossi, A.M.; Farina, M.; Andrade, L.R.; Takeuchi, A.Y.; Rocha-Leão, M.H. Microcapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulin. Colloids Surf. B 2010, 81, 206–211. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Y.; Zhao, X.; Shan, C.; Zu, S.; Wang, K.; Li, Y.; Ge, Y. Preparation and characterization of chitosan–polyvinyl alcohol blend hydrogels for the controlled release of nano-insulin. Int. J. Biol. Macromol. 2012, 50, 82–87. [Google Scholar] [CrossRef]
- Balena, R.; Hensley, I.; Miller, S.; Barnett, A. Combination therapy with GLP-1 receptor agonists and basal insulin: A systematic review of the literature. Diabetes Obes. Metab. 2013, 15, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhulkotia, J.S.; Ola, B.; Fraser, R.; Farrell, T. Oral hypoglycemic agents vs insulin in management of gestational diabetes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2010, 203, 457.e1–457.e9. [Google Scholar] [CrossRef] [PubMed]
- Jeitler, K.; Horvath, K.; Berghold, A.; Gratzer, T.; Neeser, K.; Pieber, T.; Siebenhofer, A. Continuous Subcutaneous Insulin Infusion Versus Multiple Daily Insulin Injections in Patients with Diabetes Mellitus: Systematic Review and Meta-Analysis; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Khunti, K.; Millar-Jones, D. Clinical inertia to insulin initiation and intensification in the UK: A focused literature review. Prim. Care Diabetes 2017, 11, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University: Keele, UK, 2004; Volume 33, pp. 1–26. [Google Scholar]
- Kitchenham, B.; Pretorius, R.; Budgen, D.; Brereton, O.P.; Turner, M.; Niazi, M.; Linkman, S. Systematic literature reviews in software engineering—A tertiary study. Inf. Softw. Technol. 2010, 52, 792–805. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Humenberger, C.; Valenta, C. Basic studies on bioadhesive delivery systems for peptide and protein drugs. Int. J. Pharm. 1998, 165, 217–225. [Google Scholar] [CrossRef]
- Robles, E.; Juárez, J.; Burboa, M.G.; Gutiérrez, L.E.; Taboada, P.; Mosquera, V.; Valdez, M.A. Properties of insulin–chitosan complexes obtained by an alkylation reaction on chitosan. J. Appl. Polym. Sci. 2014, 131, 6. [Google Scholar] [CrossRef]
- Li, M.-G.; Lu, W.-L.; Wang, J.-C.; Zhang, X.; Zhang, H.; Wang, X.-Q.; Wu, C.-S.; Zhang, Q. Preparation and characterization of insulin nanoparticles employing chitosan and poly (methylmethacrylate/methylmethacrylic acid) copolymer. J. Nanosci. Nanotechnol. 2006, 6, 2874–2886. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Jorgensen, L.; Van De Weert, M. Probing insulin’s secondary structure after entrapment into alginate/chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2007, 65, 10–17. [Google Scholar] [CrossRef]
- Avadi, M.R.; Sadeghi, A.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed. Nanotechnol. 2010, 6, 58–63. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Jiang, W.; Ren, C.; Li, J.; Xin, J.; Li, K. Effective protection and controlled release of insulin by cationic β-cyclodextrin polymers from alginate/chitosan nanoparticles. Int. J. Pharm. 2010, 393, 213–219. [Google Scholar] [CrossRef]
- Omid, N.J.; Babanejad, N.; Amini, H.; Amini, M.; Tehrani, M.R.; Dorkoosh, F. Preparation and characterization of novel derivatives of chitosan and trimethyl chitosan conjugated with dipeptides and vitamin B12 as candidates for oral delivery of insulin. J. Polym. Res. 2014, 21, 510. [Google Scholar] [CrossRef]
- Al-Kurdi, Z.I.; Chowdhry, B.Z.; Leharne, S.A.; Al Omari, M.M.; Badwan, A.A. Low molecular weight chitosan-insulin polyelectrolyte complex: Characterization and stability studies. Mar. Drugs 2015, 13, 1765–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yang, L.; Li, M.; Zhang, L. A cell-penetrating peptide mediated chitosan nanocarriers for improving intestinal insulin delivery. Carbohydr. Polym. 2017, 174, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Leong, K.H.; Nyamathulla, S.; Onuki, Y.; Takayama, K.; Chung, L.Y. Optimization of pH-responsive carboxymethylated iota-carrageenan/chitosan nanoparticles for oral insulin delivery using response surface methodology. React. Funct. Polym. 2017, 119, 145–155. [Google Scholar] [CrossRef]
- Ji, N.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Fabrication and characterization of complex nanoparticles based on carboxymethyl short chain amylose and chitosan by ionic gelation. Food Funct. 2018, 9, 2902–2912. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Viswanad, B.; Sharma, G.; Bhardwaj, V.; Ramarao, P.; Kumar, M.R. Design and evaluation of biodegradable, biosensitive in situ gelling system for pulsatile delivery of insulin. Biomaterials 2007, 28, 2051–2060. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.; Jon, S. A novel approach to oral delivery of insulin by conjugating with low molecular weight chitosan. Bioconjug. Chem. 2010, 21, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Balabushevich, N.G.; Pechenkin, M.A.; Shibanova, E.D.; Volodkin, D.V.; Mikhalchik, E.V. Multifunctional polyelectrolyte microparticles for oral insulin delivery. Macromol. Biosci. 2013, 13, 1379–1388. [Google Scholar] [CrossRef]
- Gu, Z.; Dang, T.T.; Ma, M.; Tang, B.C.; Cheng, H.; Jiang, S.; Dong, Y.; Zhang, Y.; Anderson, D.G. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano 2013, 7, 6758–6766. [Google Scholar] [CrossRef]
- Ghasemi Tahrir, F.; Ganji, F.; Mani, A.R.; Khodaverdi, E. In vitro and in vivo evaluation of thermosensitive chitosan hydrogel for sustained release of insulin. Drug Deliv. 2014, 23, 1038–1046. [Google Scholar] [CrossRef]
- Wang, J.; Kong, M.; Zhou, Z.; Yan, D.; Yu, X.; Cheng, X.; Feng, C.; Liu, Y.; Chen, X. Mechanism of surface charge triggered intestinal epithelial tight junction opening upon chitosan nanoparticles for insulin oral delivery. Carbohydr. Polym. 2017, 157, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Yu, J.; Wang, Q.; Yao, S.; Suo, D.; Ye, Y.; Pless, M.; Zhu, Y.; Jing, Y.; Gu, Z. Ultrasound-triggered noninvasive regulation of blood glucose levels using microgels integrated with insulin nanocapsules. Nano Res. 2017, 10, 1393–1402. [Google Scholar] [CrossRef]
- Al-Remawi, M.; Elsayed, A.; Maghrabi, I.; Hamaidi, M.; Jaber, N. Chitosan/lecithin liposomal nanovesicles as an oral insulin delivery system. Pharm. Dev. Technol. 2017, 22, 390–398. [Google Scholar] [CrossRef]
- Lopes, M.A.; Abrahim, B.A.; Cabral, L.M.; Rodrigues, C.R.; Seiça, R.M.F.; de Baptista Veiga, F.J.; Ribeiro, A.J. Intestinal absorption of insulin nanoparticles: Contribution of M cells. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Krauland, A.H.; Alonso, M.J. Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int. J. Pharm. 2007, 340, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, S.; Zhang, X.; Shu, S.; Chu, T.; Yu, D. Phenylboronic acid grafted chitosan as a glucose-sensitive vehicle for controlled insulin release. J. Pharm. Sci. 2011, 100, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Fangueiro, J.; Smitha, J.; Cinu, T.; Chacko, A.; Premaletha, K.; Souto, E. Predictive modeling of insulin release profile from cross-linked chitosan microspheres. Eur. J. Med. Chem. 2013, 60, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Tahtat, D.; Mahlous, M.; Benamer, S.; Khodja, A.N.; Oussedik-Oumehdi, H.; Laraba-Djebari, F. Oral delivery of insulin from alginate/chitosan crosslinked by glutaraldehyde. Int. J. Biol. Macromol. 2013, 58, 160–168. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Tafaghodi, M.; Ganji, F.; Abnoos, K.; Naghizadeh, H. In vitro insulin release from thermosensitive chitosan hydrogel. Aaps Pharmscitech 2012, 13, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Goycoolea, F.M.; Lollo, G.; Remunán-López, C.; Quaglia, F.; Alonso, M.J. Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef]
- Lee, D.H.; Kang, I.J. Drug delivery system using biodegradable nanoparticles carrier. Kona Powder Part. J. 2006, 24, 159–166. [Google Scholar] [CrossRef] [Green Version]
- De Villiers, M.M.; Aramwit, P.; Kwon, G.S. Nanotechnology in Drug Delivery; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Elsayed, A.; Al-Remawi, M.; Qinna, N.; Farouk, A.; Al-Sou’od, K.A.; Badwan, A.A. Chitosan–sodium lauryl sulfate nanoparticles as a carrier system for the in vivo delivery of oral insulin. Aaps Pharmscitech 2011, 12, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciel, V.B.; Yoshida, C.M.; Pereira, S.M.; Goycoolea, F.M.; Franco, T.T. Electrostatic self-assembled chitosan-pectin nano-and microparticles for insulin delivery. Molecules 2017, 22, 1707. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Grenha, A.; Carrión-Recio, D.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control. Release Off. J. Control. Release Soc. 2012, 157, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Nasim, F.; Mishra, R.; Bharti, R.P.; Kundu, P. Polyurethane-incorporated chitosan/alginate core–shell nano-particles for controlled oral insulin delivery. J. Appl. Polym. Sci. 2018, 135, 46365. [Google Scholar] [CrossRef]
- Albuquerque, D.; Castro, J.; Ribeiro, S.; Heineck, T. Requirements Engineering for Robotic System: A Systematic Mapping Study; CIbSE: London, UK, 2017; pp. 333–346. [Google Scholar]












| RQ | Description | Motivation |
|---|---|---|
| RQ1 | At what phase is the development of the chitosan/insulin delivery system? | This question provides a starting point to investigate the temporal progress of the development of chitosan/insulin release systems. |
| RQ2 | What are the release systems developed? | The aim was to classify and identify the models developed from 1998 to 2018. |
| RQ3 | What are the main alternative ways of administration suggested? | This question aims to identify the alternative administration forms proposed. |
| RQ4 | What is the total amount of insulin loaded and the encapsulation efficiency of the release system? | This question intends to investigate the efficiency of the methods used to prepare the delivery system. |
| RQ5 | For how long did the system release insulin? | The answer to this question allows knowledge of how long the systems can control glucose levels. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, F.C.; Silva, M.C.d.; Silva, H.N.d.; Albuquerque, D.; Gomes, A.A.R.; Silva, S.M.d.L.; Fook, M.V.L. Progress in the Development of Chitosan Based Insulin Delivery Systems: A Systematic Literature Review. Polymers 2020, 12, 2499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112499
Barbosa FC, Silva MCd, Silva HNd, Albuquerque D, Gomes AAR, Silva SMdL, Fook MVL. Progress in the Development of Chitosan Based Insulin Delivery Systems: A Systematic Literature Review. Polymers. 2020; 12(11):2499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112499
Chicago/Turabian StyleBarbosa, Francivandi Coelho, Milena Costa da Silva, Henrique Nunes da Silva, Danyllo Albuquerque, Allyson Antônio Ribeiro Gomes, Suédina Maria de Lima Silva, and Marcus Vinícius Lia Fook. 2020. "Progress in the Development of Chitosan Based Insulin Delivery Systems: A Systematic Literature Review" Polymers 12, no. 11: 2499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112499