Investigating the Impact of Curing System on Structure-Property Relationship of Natural Rubber Modified with Brewery By-Product and Ground Tire Rubber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
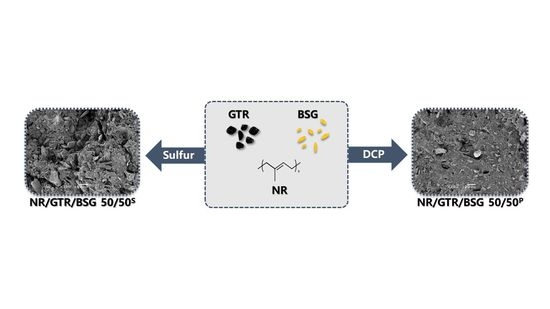
2.2. Sample Preparation
2.3. Measurements
3. Results
3.1. Curing Characteristics
3.2. FTIR Analysis
3.3. Physico-Mechanical Properties
3.4. Thermogravimetric Analysis
3.5. Scanning Electron Microscopy
3.6. Acoustic Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaaba, N.F.; Ismail, H.; Jaafar, M. Effect of peanut shell powder content on the properties of recycled polypropylene (RPP)/peanut shell powder (PSP) composites. BioResources 2013, 8, 5826–5841. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.S.; Kim, H.J.; Son, J.; Park, H.J.; Lee, B.J.; Hwang, T.S. Rice-husk flour filled polypropylene composites; mechanical and morphological study. Compos. Struct. 2004, 63, 305–312. [Google Scholar] [CrossRef]
- Akil, H.M.; Omar, M.F.; Mazuki, A.A.M.; Safiee, S.; Ishak, Z.A.M.; Abu Bakar, A. Kenaf fibre reinforced composites: A review. Mater. Des. 2011, 32, 4107–4121. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, S. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part A Appl. Sci. Manuf. 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Sareena, C.; Ramesan, M.T.; Purushothaman, E. Utilization of peanut shell powder as a novel filler in natural rubber. J. Appl. Polym. Sci. 2012, 125, 2322–2334. [Google Scholar] [CrossRef]
- Jamil, M.S.; Ahmad, I.; Abdullah, I. Effects of rice husk filler on the mechanical and thermal properties of liquid natural rubber compatibilized high-density polyethylene/natural rubber blends. J. Polym. Res. 2006, 13, 315–321. [Google Scholar] [CrossRef]
- Ikeda, Y.; Phakkeeree, T.; Junkong, P.; Yokohama, H.; Phinyocheep, P.; Kitano, R.; Kato, A. Reinforcing biofiller “lignin” for high performance green natural rubber nanocomposites. RSC Adv. 2017, 7, 5222–5231. [Google Scholar] [CrossRef] [Green Version]
- Ketabchi, M.R.; Ratnam, C.T.; Khalid, M.; Walvekar, R. Mechanical properties of polylactic acid/synthetic rubber blend reinforced with cellulose nanoparticles isolated from kenaf fibres. Polym. Bull. 2018, 75, 809–827. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, M.; Chen, L. Interface and bonding mechanisms of plant fibre composites: An overview. Compos. Part B Eng. 2016, 101, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Reihmane, S.; Gassan, J. Thermoplastics reinforced with wood fillers: A literature review. Polym. Plast. Technol. Eng. 1998, 37, 451–468. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kato, A.; Kohjiya, S.; Nakajima, Y. Rubber Science: A Modern Approach; Springer: Berlin, Germany, 2017. [Google Scholar]
- Colom, X.; Carrasco, F.; Pagès, P.; Cañavate, J. Effects of different treatments on the interface of HDPE/lignocellulosic fiber composites. Compos. Sci. Technol. 2003, 63, 161–169. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Gandini, A. The surface modification of cellulose fibres for use as reinforcing elements in composite materials. Compos. Interfaces 2005, 12, 41–75. [Google Scholar] [CrossRef]
- Gallos, A.; Paës, G.; Allais, F.; Beaugrand, J. Lignocellulosic fibers: A critical review of the extrusion process for enhancement of the properties of natural fiber composites. RSC Adv. 2017, 7, 34638–34654. [Google Scholar] [CrossRef]
- Lu, T.; Liu, S.; Jiang, M.; Xu, X.; Wang, Y.; Wang, Z.; Gou, J.; Hui, D.; Zhou, Z. Effects of modifications of bamboo cellulose fibers on the improved mechanical properties of cellulose reinforced poly(lactic acid) composites. Compos. Part B Eng. 2014, 62, 191–197. [Google Scholar] [CrossRef]
- Simon, D.Á.; Pirityi, D.; Tamás-Bényei, P.; Bárány, T. Microwave devulcanization of ground tire rubber and applicability in SBR compounds. J. Appl. Polym. Sci. 2020, 137, 48351. [Google Scholar] [CrossRef] [Green Version]
- Cañavate, J.; Colom, X.; Saeb, M.R.; Przybysz, M.; Zedler, L.; Formela, K. Influence of microwave treatment conditions of GTR on physico-mechanical and structural properties of NBR/NR/GTR composites. Afinidad 2019, 76, 171–179. [Google Scholar]
- Wu, D.Y.; Bateman, S.; Partlett, M. Ground rubber/acrylonitrile-butadiene-styrene composites. Compos. Sci. Technol. 2007, 67, 1909–1919. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, C.; Liang, M. Properties of natural rubber vulcanizates containing mechanochemically devulcanized ground tire rubber. J. Polym. Res. 2009, 16, 411–419. [Google Scholar] [CrossRef]
- Fukumori, K.; Matsushita, M.; Okamoto, H.; Sato, N.; Suzuki, Y.; Takeuchi, K. Recycling technology of tire rubber. JSAE Rev. 2002, 23, 259–264. [Google Scholar] [CrossRef]
- Barrera, C.S.; Cornish, K. High performance waste-derived filler/carbon black reinforced guayule natural rubber composites. Ind. Crops Prod. 2016, 86, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Suttivutnarubet, C.; Jaturapiree, A.; Chaichana, E.; Praserthdam, P.; Jongsomjit, B. Synthesis of polyethylene/coir dust hybrid filler via in situ polymerization with zirconocene/MAO catalyst for use in natural rubber biocomposites. Iran. Polym. J. 2016, 25, 841–848. [Google Scholar] [CrossRef]
- Yantaboot, K.; Amornsakchai, T. Effect of preparation methods and carbon black distribution on mechanical properties of short pineapple leaf fiber-carbon black reinforced natural rubber hybrid composites. Polym. Test. 2017, 61, 223–228. [Google Scholar] [CrossRef]
- Abdul Salim, Z.A.S.; Hassan, A.; Ismail, H. A review on hybrid fillers in rubber composites. Polym. Plast. Technol. Eng. 2018, 57, 523–539. [Google Scholar] [CrossRef]
- Zedler, Ł.; Colom, X.; Saeb, M.R.; Formela, K. Preparation and characterization of natural rubber composites highly filled with brewers’ spent grain/ground tire rubber hybrid reinforcement. Compos. Part B Eng. 2018, 145, 182–188. [Google Scholar] [CrossRef]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler-filler and filler-elastomer interaction on rubber reinforcement. Compos. Part A Appl. Sci. Manuf. 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, I.-S.; Woo, C.-S. Influence of TESPT content on crosslink types and rheological behaviors of natural rubber compounds reinforced with silica. J. Appl. Polym. Sci. 2007, 106, 2753–2758. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Dierkes, W.K.; Noordermeer, J.W.M. Elucidation of filler-to-filler and filler-to-rubber interactions in silica-reinforced natural rubber by TEM Network Visualization. Eur. Polym. J. 2014, 54, 118–127. [Google Scholar] [CrossRef]
- Sae-Oui, P.; Rakdee, C.; Thanmathorn, P. Use of rice husk ash as filler in natural rubber vulcanizates: In comparison with other commercial fillers. J. Appl. Polym. Sci. 2002, 83, 2485–2493. [Google Scholar] [CrossRef]
- Hernández, M.; Valentín, J.L.; López-Manchado, M.A.; Ezquerra, T.A. Influence of the vulcanization system on the dynamics and structure of natural rubber: Comparative study by means of broadband dielectric spectroscopy and solid-state NMR spectroscopy. Eur. Polym. J. 2015, 68, 90–103. [Google Scholar] [CrossRef]
- Kruzelák, J.; Dosoudil, R.; SÝKora, R.; Hudec, I. Rubber composites cured with sulphur and peroxide and incorporated with strontium ferrite. Bull. Mater. Sci. 2017, 40, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization of rubber compounds with peroxide curing systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Rattanasom, N.; Poonsuk, A.; Makmoon, T. Effect of curing system on the mechanical properties and heat aging resistance of natural rubber/tire tread reclaimed rubber blends. Polym. Test. 2005, 24, 728–732. [Google Scholar] [CrossRef]
- El-Nemr, K.F. Effect of different curing systems on the mechanical and physico-chemical properties of acrylonitrile butadiene rubber vulcanizates. Mater. Des. 2011, 32, 3361–3369. [Google Scholar] [CrossRef]
- Li, S.; Lamminmäki, J.; Hanhi, K. Improvement of mechanical properties of rubber compounds using waste rubber/virgin rubber. Polym. Eng. Sci. 2005, 45, 1239–1246. [Google Scholar] [CrossRef]
- Formela, K.; Cysewska, M.; Haponiuk, J.T. Thermomechanical reclaiming of ground tire rubber via extrusion at low temperature: Efficiency and limits. J. Vinyl Addit. Technol. 2016, 22, 213–221. [Google Scholar] [CrossRef]
- Hejna, A.; Formela, K.; Saeb, M.R. Processing, mechanical and thermal behavior assessments of polycaprolactone/agricultural wastes biocomposites. Ind. Crop. Prod. 2015, 76, 725–733. [Google Scholar] [CrossRef]
- Sreeja, T.D.; Kutty, S.K.N. Studies on acrylonitrile butadiene rubber/reclaimed rubber blends. J. Elastom. Plast. 2002, 34, 145–155. [Google Scholar] [CrossRef]
- Ramezani Kakroodi, A.; Kazemi, Y.; Rodrigue, D. Mechanical, rheological, morphological and water absorption properties of maleated polyethylene/hemp composites: Effect of ground tire rubber addition. Compos. Part B Eng. 2013, 51, 337–344. [Google Scholar] [CrossRef]
- Colom, X.; Marín-Genesca, M.; Mujal, R.; Formela, K.; Cañavate, J. Structural and physico-mechanical properties of natural rubber/GTR composites devulcanized by microwaves: Influence of GTR source and irradiation time. J. Compos. Mater. 2018, 52, 3099–3108. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds‡–overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- Albouy, P.A.; Sotta, P. Strain-induced crystallization in natural rubber. Adv. Polym. Sci. 2017, 277, 167–206. [Google Scholar]
- Nie, Y. Strain-induced crystallization of natural rubber/zinc dimethacrylate composites studied using synchrotron X-ray diffraction and molecular simulation. J. Polym. Res. 2015, 22, 1. [Google Scholar] [CrossRef]
- Brüning, K.; Schneider, K.; Roth, S.V.; Heinrich, G. Kinetics of strain-induced crystallization in natural rubber studied by WAXD: Dynamic and impact tensile experiments. Macromolecules 2012, 45, 7914–7919. [Google Scholar] [CrossRef]
- Ozbas, B.; Toki, S.; Hsiao, B.S.; Chu, B.; Register, R.A.; Aksay, I.A.; Prud’Homme, R.K.; Adamson, D.H. Strain-induced crystallization and mechanical properties of functionalized graphene sheet-filled natural rubber. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 718–723. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood. Part 1. Weight loss kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Carrillo, F.; Lis, M.J. Acoustic and mechanical properties of recycled polyvinyl chloride/ground tyre rubber composites. J. Compos. Mater. 2014, 48, 1061–1069. [Google Scholar] [CrossRef]








| Component | Abbreviation | Function | Characteristics | Company | Treatment before Use |
|---|---|---|---|---|---|
| Natural rubber | NR | matrix | Ribbed Smoked Sheets type with density 0.92 g/cm3 | Guma-Pomorska (Głobino, Poland) | Used as received. |
| Ground tire rubber | GTR | filler | Particles size: below 0.8 mm | Orzeł S.A. (Poniatowa, Poland) | Prior to processing, GTR was mechano-chemically reclaimed at ambient temperature using two-roll mills. GTR in presence of bitumen was masticated for 10 min (after this time homogenous material was formed), in order to enhance matrix-filler interactions [36,37]. |
| Brewer’s spent grain | BSG | filler | Initial composition: wheat malts 47.7 wt.% and barley malts 52.3 wt.% Particles size: below 0.8 mm | Dno Bojlera (Rotmanka, Poland) | Prior to processing, BSG was dried at 80 °C and then mechanically grounded in a co-rotating twin-screw extruder at 120 °C in order to obtain particles with a similar size distribution. |
| N-tert-butyl-2-benzothiazole sulfonamide | TBBS | accelerator | Molar mass: 238.37 g/mol Density: 1.29 g/cm3 Melting temperature: 104–111 °C | Standard Sp. z o.o. (Lublin, Poland) | Used as received. |
| tetramethylthiuram disulfide | TMTD | accelerator | Molar mass: 240.43 g/mol Density: 1.38 g/cm3 Melting temperature: 146–148 °C | Standard Sp. z o.o. (Lublin, Poland) | Used as received. |
| Stearic acid | St | plasticizer/activator | Molar mass: 284.48 g/mol Density: 0.94 g/cm3 Melting temperature: 69.3 °C | Standard Sp. z o.o. (Lublin, Poland) | Used as received. |
| Zinc oxide | ZnO | activator | Molar mass: 81.38 g/mol Density: 5.61 g/cm3 | Standard Sp. z o.o. (Lublin, Poland) | Used as received. |
| Sulfur | S | radicals initiator | Density: 2.07 g/cm3 Melting temperature: 115 °C | Standard Sp. z o.o. (Lublin, Poland) | Used as received. |
| Dicumyl peroxide | DCP | radicals initiator | Molar mass: 270.37 g/mol Density: 1.56 g/cm3 Melting temperature: 39–41 °C | Pergan GmbH (Bocholt, Germany) | Used as received. |
| Components (phr) | NR/BSG100S | NR/BSG/GTR50/50S | NR/GTR100S | NR/BSG100P | NR/BSG/GTR50/50P | NR/GTR100P |
|---|---|---|---|---|---|---|
| NR | 100 | 100 | 100 | 100 | 100 | 100 |
| GTR | 0 | 50 | 100 | 0 | 50 | 100 |
| BSG | 100 | 50 | 0 | 100 | 50 | 0 |
| Curing characteristics at 160 °C (sulfur system) | Curing characteristics at 180 °C (DCP) | |||||
| Minimal torque (dNm) | 1.7 | 5.0 | 4.8 | 1.9 | 1.8 | 3.8 |
| Maximal torque (dNm) | 41.3 | 30.9 | 23.6 | 45.9 | 28.6 | 20.0 |
| ΔM (dNm) | 39.6 | 25.9 | 18.8 | 44.0 | 26.8 | 16.2 |
| Scorch time (t2, min) | 3.9 | 2.0 | 2.6 | 1.3 | 1.3 | 1.3 |
| Optimum cure time (t90, min) | 10.1 | 6.4 | 5.7 | 4.1 | 4.1 | 4.1 |
| Cure rate index (CRI. Min−1) | 16.1 | 22.5 | 31.8 | 34.6 | 35.5 | 35.6 |
| Thermal aging resistance (R300%) | 0.5 | 0.6 | 1.1 | 1.6 | 1.3 | 1.9 |
| Components (phr) | NR/BSG100S | NR/BSG/GTR50/50S | NR/GTR100S | NR/BSG100P | NR/BSG/GTR50/50P | NR/GTR100P |
|---|---|---|---|---|---|---|
| NR | 100 | 100 | 100 | 100 | 100 | 100 |
| GTR | 0 | 50 | 100 | 0 | 50 | 100 |
| BSG | 100 | 50 | 0 | 100 | 50 | 0 |
| Physico-mechanical properties | ||||||
| Tensile strength (MPa) | 4.5 ± 0.1 | 6.2 ± 1.4 | 8.0 ± 0.4 | 2.7 ± 0.1 | 3.9 ± 0.7 | 3.3 ± 0.7 |
| Elongation at break (%) | 475 ± 12 | 452 ± 84 | 511 ± 27 | 221 ± 48 | 341 ± 56 | 302 ± 78 |
| M100 (MPa) | 1.8 ± 0.1 | 1.5 ± 0.2 | 0.9 ± 0.1 | 2.1 ± 0.1 | 1.2 ± 0.1 | 0.8 ± 0.1 |
| Hardness (Sh A) | 66 ± 1 | 53 ± 1 | 44 ± 1 | 63 ± 1 | 48 ± 1 | 35 ± 1 |
| Density at 25 °C (g/cm3) | 1.11 ± 0.01 | 1.08 ± 0.01 | 1.04 ± 0.01 | 1.09 ± 0.02 | 1.06 ± 0.01 | 1.02 ± 0.01 |
| Sol fraction (%) | 3.38 ± 0.20 | 5.34 ± 0.20 | 7.58 ± 0.24 | 4.52 ± 0.03 | 6.58 ± 0.20 | 8.76 ± 0.19 |
| Swelling degree (%) | 232 ± 2 | 228 ± 6 | 277 ± 4 | 141 ± 2 | 232 ± 4 | 307 ± 2 |
| Cross-link density (mol/cm3 × 10−4) | 1.80 ± 0.03 | 0.87 ± 0.02 | 0.62 ± 0.01 | 1.89 ± 0.04 | 0.85 ± 0.03 | 0.53 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedler, Ł.; Colom, X.; Cañavate, J.; Saeb, M.R.; T. Haponiuk, J.; Formela, K. Investigating the Impact of Curing System on Structure-Property Relationship of Natural Rubber Modified with Brewery By-Product and Ground Tire Rubber. Polymers 2020, 12, 545. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12030545
Zedler Ł, Colom X, Cañavate J, Saeb MR, T. Haponiuk J, Formela K. Investigating the Impact of Curing System on Structure-Property Relationship of Natural Rubber Modified with Brewery By-Product and Ground Tire Rubber. Polymers. 2020; 12(3):545. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12030545
Chicago/Turabian StyleZedler, Łukasz, Xavier Colom, Javier Cañavate, Mohammad Reza Saeb, Józef T. Haponiuk, and Krzysztof Formela. 2020. "Investigating the Impact of Curing System on Structure-Property Relationship of Natural Rubber Modified with Brewery By-Product and Ground Tire Rubber" Polymers 12, no. 3: 545. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12030545