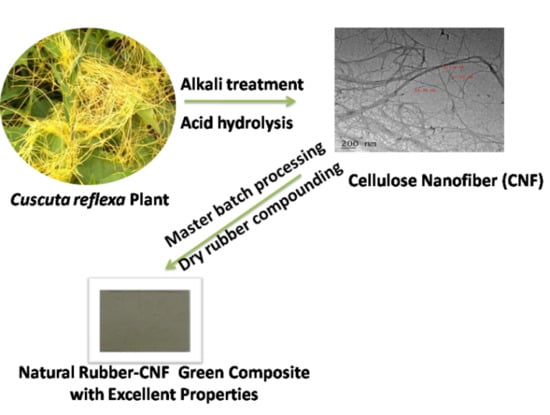
Cellulose Nanofibers Isolated from the Cuscuta Reflexa Plant as a Green Reinforcement of Natural Rubber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Cellulosic Nanofibers
2.2.2. Preparation of NR–CNF Nanocomposites
2.2.3. Characterization Techniques
3. Results and Discussion
3.1. Chemical Composition of CNF
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. X-Ray Diffraction (XRD)
3.4. Scanning Electron Microscopy (SEM)
3.5. Transmission Electron Microscopy (TEM)
3.6. Thermogravimetric Analysis of CNF
3.7. Cure Characteristics of NR–CNF Nanocomposites
3.8. Mechanical Properties of NR–CNF Nanocomposites
3.9. Swelling Studies of NR–CNF Nanocomposites
3.10. Fractographic Studies of NR–CNF Nanocomposites
3.11. Thermogravimetric Analysis of NR–CNF Composites
3.12. Dynamic Mechanical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arayapranee, W.; Naranong, N.; Rempel, G.L. Application of rice husk ash as fillers in the natural rubber industry. J. Appl. Polym. Sci. 2005, 98, 34–41. [Google Scholar] [CrossRef]
- Ahmed, K.; Nizami, S.S.; Riza, N.Z. Reinforcement of natural rubber hybrid composites based on marble sludge/Silica and marble sludge/rice husk derived silica. J. Adv. Res. 2014, 5, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, M.; Thomas, S.; Varughese, K.T. Natural rubber composites reinforced with sisal/oil palm hybrid fibers: Tensile and cure characteristics. J. Appl. Polym. Sci. 2004, 93, 2305–2312. [Google Scholar] [CrossRef]
- Pangamol, P.; Malee, W.; Yujaroen, R.; Sae-Oui, P.; Siriwong, C. Utilization of bagasse ash as a filler in natural rubber and styrene–butadiene rubber composites. Arab. J. Sci. Eng. 2018, 43, 221–227. [Google Scholar] [CrossRef]
- Visakh, P.M. Rubber Based Bionanocomposites: Preparation; Springer Nature: Cham, Switzerland, 2017. [Google Scholar]
- Bao, C.A.; Kamaruddin, S.; Yeow, T.K.; Ing, K.; Tay, B.; Han, J. The effect of oil palm fiber / eggshell powder loading on the mechanical properties of natural rubber composites. ARPN J. Eng. Appl. Sci. 2016, 11, 128–134. [Google Scholar]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- John, S.; Joseph, R.; Issac, J.M. Mechanical and cure characteristics of natural rubber composites with caryota fibre incorporated in dry stage and latex stage. Appl. Mech. Mater. 2015, 766–767, 100–103. [Google Scholar] [CrossRef]
- Joseph, S.; Joseph, K.; Thomas, S. Green composites from natural rubber and oil palm fiber: Physical and mechanical properties. Int. J. Polym. Mater. 2006, 55, 925–945. [Google Scholar] [CrossRef]
- Pittayavinai, P.; Thanawan, S.; Amornsakchai, T. Comparative study of natural rubber and acrylonitrile rubber reinforced with aligned short aramid fiber. Polym. Test. 2017, 64, 109–116. [Google Scholar] [CrossRef]
- Visakh, P.M.; Thomas, S.; Oksman, K.; Mathew, A.P. Cellulose nanofibres and cellulose nanowhiskers based natural rubber composites: Diffusion, sorption, and permeation of aromatic organic solvents. J. Appl. Polym. Sci. 2011, 124, 1614–1623. [Google Scholar] [CrossRef]
- Natinee, L.; Dolmalik, J.; Manus, S. Hybridized reinforcement of natural rubber with silane-modified short cellulose fibers and silica. J. Appl. Polym. Sci. 2011, 120, 3242–3254. [Google Scholar]
- Formela, K.; Hejna, A.; Piszczyk, Ł.; Saeb, M.R.; Colom, X. Processing and structure–property relationships of natural rubber/wheat bran biocomposites. Cellulose 2016, 23, 3157–3175. [Google Scholar] [CrossRef]
- Mathew, L.; Joseph, K.U.; Joseph, R. Swelling behaviour of isora/natural rubber composites in oils used in automobiles. Bull. Mater. Sci. 2006, 29, 91–99. [Google Scholar] [CrossRef]
- Fumagalli, M.; Berriot, J.; De Gaudemaris, B.; Veyland, A.; Putaux, J.L.; Molina-Boisseau, S.; Heux, L. Rubber materials from elastomers and nanocellulose powders: Filler dispersion and mechanical reinforcement. Soft Matter 2018, 14, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Parambath Kanoth, B.; Claudino, M.; Johansson, M.; Berglund, L.A.; Zhou, Q. Biocomposites from natural rubber: Synergistic effects of functionalized cellulose nanocrystals as both reinforcing and cross-linking agents via free-radical thiol-ene chemistry. ACS Appl. Mater. Interfaces 2015, 7, 16303–16310. [Google Scholar] [CrossRef] [PubMed]
- Dominic, M.; Joseph, R.; Sabura Begum, P.M.; Kanoth, B.P.; Chandra, J.; Thomas, S. Green tire technology: Effect of rice husk derived nanocellulose (RHNC) in replacing carbon black (CB) in natural rubber (NR) compounding. Carbohydr. Polym. 2020, 230, 115620. [Google Scholar] [CrossRef] [PubMed]
- Flauzino Neto, W.P.; Mariano, M.; da Silva, I.S.V.; Silvério, H.A.; Putaux, J.L.; Otaguro, H.; Pasquini, D.; Dufresne, A. Mechanical properties of natural rubber nanocomposites reinforced with high aspect ratio cellulose nanocrystals isolated from soy hulls. Carbohydr. Polym. 2016, 153, 143–152. [Google Scholar] [CrossRef]
- Han, J.; Lu, K.; Yue, Y.; Mei, C.; Huang, C.; Wu, Q.; Xu, X. Nanocellulose-templated assembly of polyaniline in natural rubber-based hybrid elastomers toward flexible electronic conductors. Ind. Crop. Prod. 2019, 128, 94–107. [Google Scholar] [CrossRef]
- Kaiser, B.; Vogg, G.; Fürst, U.B.; Albert, M. Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Cherian, B.M.; Pothan, L.A.; Nguyen-chung, T.; Mennig, G.; Kottaisamy, M.; Thomas, S. A Novel Method for the Synthesis of Cellulose Nanofibril Whiskers from Banana Fibers and Characterization. J. Agric. Food Chem. 2008, 56, 5617–5627. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Sonia, A.; Priya Dasan, K. Chemical, morphology and thermal evaluation of cellulose microfibers obtained from Hibiscus sabdariffa. Carbohydr. Polym. 2013, 92, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J.; Rehner, J. Statistical mechanics of crosslinked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Ellis, B.; Welding, G.N. Estimation, from swelling, of the structural contribution of chemical reactions to the vulcanization of natural rubber. Part II. Estimation of equilibrium degree of swelling. Rubber Chem. Technol. 1964, 37, 571–575. [Google Scholar] [CrossRef]
- Kalita, E.; Nath, B.K.; Agan, F.; More, V.; Deb, P. Isolation and characterization of crystalline, autofluorescent, cellulose nanocrystals from saw dust wastes. Ind. Crop. Prod. 2015, 65, 550–555. [Google Scholar] [CrossRef]
- Mano, B.; Araujo, J.R.; De Paoli, M.-A.; Waldman, W.R.; Spinace, M.A. Mechanical properties, morphology and thermal degradation of a biocomposite of polypropylene and curaua fibers: Coupling agent effect. Polímeros Ciência e Tecnologia 2013, 23, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Ludueña, L.; Fasce, D.; Alvarez, V.A.; Stefani, P.M. Nanocellulose from rice husk following alkaline treatment to remove silica. BioResources 2011, 6, 1440–1453. [Google Scholar]
- Abraham, E.; Deepa, B.; Pothen, L.A.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef]
- Astruc, J.; Nagalakshmaiah, M.; Laroche, G.; Grandbois, M.; Elkoun, S.; Robert, M. Isolation of cellulose-II nanospheres from flax stems and their physical and morphological properties. Carbohydr. Polym. 2017, 178, 352–359. [Google Scholar] [CrossRef]
- Rodrigues, J.; Faix, O.; Pereira, H. Determination of lignin content of Eucalyptus globulus wood using FTIR spectroscopy. Holzforschung 1998, 52, 46–50. [Google Scholar] [CrossRef]
- Rani, A.; Monga, S.; Bansal, M.; Sharma, A. Bionanocomposites reinforced with cellulose nanofibers derived from sugarcane bagasse. Polym. Compos. 2018, 39, E55–E64. [Google Scholar] [CrossRef]
- Mora, J.I.; Alvarez, V.A.; Cyras, V.P.; Vazquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Spinacé, M.A.S.; Lambert, C.S.; Fermoselli, K.K.G.; De Paoli, M.A. Characterization of lignocellulosic curaua fibres. Carbohydr. Polym. 2009, 77, 47–53. [Google Scholar] [CrossRef]
- Prasad Reddy, J.; Rhim, J.W. Isolation and characterization of cellulose nanocrystals from garlic skin. Mater. Lett. 2014, 129, 20–23. [Google Scholar] [CrossRef]
- Geethamma, V.G.; Joseph, R.; Thomas, S. Short coir fiber-reinforced natural-rubber composites—Effects of fiber length, orientation, and alkali treatment. J. Appl. Polym. Sci. 1995, 55, 583–594. [Google Scholar] [CrossRef]
- Kalita, E.; Nath, B.K.; Deb, P.; Agan, F.; Islam, M.R.; Saikia, K. High quality fluorescent cellulose nanofibers from endemic rice husk: Isolation and characterization. Carbohydr. Polym. 2015, 122, 308–313. [Google Scholar] [CrossRef]
- Sae-Oui, P.; Rakdee, C.; Thanmathorn, P. Use of rice husk ash as filler in natural rubber vulcanizates: In comparison with other commercial fillers. J. Appl. Polym. Sci. 2002, 83, 2485–2493. [Google Scholar] [CrossRef]
- Omofuma, F.E.; Adeniye, S.A.; Adeleke, A.E. The effect of particle sizes on the performance of filler: A case study of rice husk and wood flour. World Appl. Sci. J. 2011, 14, 1347–1352. [Google Scholar]
- Pantamanatsopa, P.; Ariyawiriyanan, W.; Meekeaw, T.; Suthamyong, R.; Arrub, K.; Hamada, H. Effect of modified jute fiber on mechanical properties of Green rubber composite. Energy Procedia 2014, 56, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.P.; Amma, M.G.; Sabu, T. Short sisal fiber reinforced styrene butadiene rubber composites. J. Appl. Polym. Sci. 1995, 58, 597–612. [Google Scholar] [CrossRef]
- Martins, A.F.; Suarez, J.C.M.; Visconte, L.L.Y.; Nunes, R.C.R. Mechanical and fractographic behavior of natural rubber-cellulose II composites. J. Mater. Sci. 2003, 38, 2415–2422. [Google Scholar] [CrossRef]
- Murty, V.M.; De, S.K. Effect of particulate fillers on short jute fiber-reinforced natural rubber composites. J. Appl. Polym. Sci. 1982, 27, 4611–4622. [Google Scholar] [CrossRef]
- Abraham, E.; Thomas, M.S.; John, C.; Pothen, L.A.; Shoseyov, O.; Thomas, S. Green nanocomposites of natural rubber/nanocellulose: Membrane transport, rheological and thermal degradation characterisations. Ind. Crop. Prod. 2013, 51, 415–424. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; John, M.; Narine, S.S.; Thomas, S.; Anandjiwala, R. Physicomechanical properties of nanocomposites based on cellulose nanofibre and natural rubber latex. Cellulose 2013, 20, 417–427. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Viscoelastic behavior and reinforcement mechanism in rubber nanocomposites in the vicinity of spherical nanoparticles. J. Phys. Chem. B 2013, 117, 12632–12648. [Google Scholar] [CrossRef]
- Intharapat, P.; Kongnoo, A.; Kateungngan, K. The potential of chicken eggshell waste as a bio-filler filled epoxidized natural rubber (ENR) composite and its properties. J. Polym. Environ. 2013, 21, 245–258. [Google Scholar] [CrossRef]
- Correia, C.A.; de Oliveira, L.M.; Valera, T.S.; Correia, C.A.; de Oliveira, L.M.; Valera, T.S. The Influence of bleached jute fiber filler on the properties of vulcanized natural rubber. Mater. Res. 2017, 20, 466–471. [Google Scholar] [CrossRef]
- Visakh, P.M.; Thomas, S.; Oksman, K.; Mathew, A.P. Crosslinked natural rubber nanocomposites reinforced with cellulose whiskers isolated from bamboo waste: Processing and mechanical/thermal properties. Compos. Part A Appl. Sci. Manuf. 2012, 43, 735–741. [Google Scholar] [CrossRef]
- Gopalan Nair, K.; Dufresne, A. Crab shell chitin whisker reinforced natural rubber. Biomacromolecules 2003, 4, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Appukuttan, S.P.; Kenny, J.M.; Puglia, D.; Thomas, S.; Joseph, K. Dynamic mechanical properties of oil palm microfibril-reinforced natural rubber composites. J. Appl. Polym. Sci. 2010, 117, 1298–1308. [Google Scholar] [CrossRef]
- Prasertsri, S.; Rattanasom, N. Fumed and precipitated silica reinforced natural rubber composites prepared from latex system: Mechanical and dynamic properties. Polym. Test. 2012, 31, 593–605. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Wang, Y.; Liu, Y.; Liu, Y.; Chen, Y. New nanocomposite materials reinforced with cellulose nanocrystals in nitrile rubber. Polym. Test. 2013, 32, 819–826. [Google Scholar] [CrossRef]
- Cao, X.; Dong, H.; Li, C.M. New nanocomposite materials reinforced with cellulose nanocrystals in waterborne polyurethane. Biomacromolecules 2007, 8, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Dileep, P.; Varghese, G.A.; Sivakumar, S.; Narayanankutty, S.K. An innovative approach to utilize waste silica fume from zirconia industry to prepare high performance natural rubber composites for multi-functional applications. Polym. Test. 2020, 81, 106172. [Google Scholar] [CrossRef]








| Sample Code | Solid NR (phr) | Masterbatch | ZnO (phr) | Stearic Acid (phr) | Nonox SP (phr) | CBS (phr) | Sulphur (phr) | |
|---|---|---|---|---|---|---|---|---|
| NR (phr) | CNF (phr) | |||||||
| NR-gum | 50 | 50 | - | 5 | 2 | 1 | 0.6 | 2.5 |
| NR–CNF 1 phr | 50 | 50 | 1 | 5 | 2 | 1 | 0.6 | 2.5 |
| NR–CNF 2 phr | 50 | 50 | 2 | 5 | 2 | 1 | 0.6 | 2.5 |
| NR–CNF 3 phr | 50 | 50 | 3 | 5 | 2 | 1 | 0.6 | 2.5 |
| NR–CNF 4 phr | 50 | 50 | 4 | 5 | 2 | 1 | 0.6 | 2.5 |
| Samples | Hemicellulose (%) | Cellulose (%) | Lignin (%) |
|---|---|---|---|
| raw Cuscuta reflexa | 21 ± 4 | 41 ± 5 | 19 ± 3 |
| alkali-treated Cuscuta reflexa | 14 ± 4 | 74 ± 5 | 12 ± 4 |
| CNF | 12 ± 5 | 78 ± 5 | 10 ± 3 |
| Properties | NR-Gum | NR–CNF 1 phr | NR–CNF 2 phr | NR–CNF 3 phr | NR–CNF 4 phr |
|---|---|---|---|---|---|
| Scorch time, ts2(min) | 2.49 | 3.27 | 3.26 | 3.25 | 3.07 |
| Optimum cure time, t90(min) | 7.42 | 8.16 | 8.16 | 8.42 | 8.19 |
| Cure rate index (min−1) | 20.28 | 20.44 | 20.40 | 19.34 | 19.76 |
| Minimum torque(ML, dNm) | 0.15 | 0.20 | 0.20 | 0.25 | 0.16 |
| Maximum torque(MH, dNm) | 6.71 | 6.96 | 7.24 | 7.47 | 7.31 |
| Differential torque, MH–ML (dNm) | 6.56 | 6.76 | 7.04 | 7.22 | 7.15 |
| Wolff activity coefficient | - | 3.04 | 3.65 | 3.35 | 2.24 |
| Properties | NR-Gum | NR–CNF 1 phr | NR–CNF 2 phr | NR–CNF 3 phr | NR–CNF 4 phr |
|---|---|---|---|---|---|
| Tensile strength (MPa) | 20.38 ± 0.44 | 20.50 ± 0.5 | 22.78 ± 0.52 | 22.28 ± 0.42 | 18.27 ± 0.38 |
| Modulus at 300% elongation (MPa) | 2.11 ± 0.04 | 2.25 ± 0.02 | 2.70 ± 0.05 | 2.90 ± 0.1 | 2.40 ± 0.12 |
| Elongation at break (%) | 810 ± 0 | 819 ± 9 | 799 ± 11 | 778 ± 10 | 763 ± 12 |
| Tear strength (N/mm) | 33.12 ± 2.51 | 34.04 ± 1.12 | 40.77 ± 1.11 | 37.58 ± 1.2 | 35.49 ± 1.09 |
| Hardness (Shore A) | 37 ± 1 | 38 ± 1 | 41 ± 1 | 41 ± 1 | 42 ± 1 |
| Compression set (%) | 3.21 ± 0.25 | 3.27 ± 0.23 | 3.27 ± 0.26 | 3.55 ± 0.28 | 3.85 ± 0.21 |
| Rebound resilience (%) | 77 ± 2 | 78 ± 3 | 79 ± 2 | 77 ± 4 | 77 ± 3 |
| Abrasion resistance index, ARI (%) | 77 ± 3 | 78 ± 1 | 80 ± 1 | 79 ± 1 | 78 ± 2 |
| Properties | NR-Gum | NR- CNF 1 phr | NR–CNF 2 phr | NR–CNF 3 phr | NR–CNF 4 phr |
|---|---|---|---|---|---|
| Swelling index (%) | 382 | 380 | 368 | 370 | 375 |
| Cross-link density (×10−5 mol/g) | 3.11 | 5.26 | 5.72 | 5.37 | 5.36 |
| Molar mass between cross-links (g/mol) | 16077 | 9505 | 8741 | 9310 | 9328 |
| ΔG (J/mol) | −7.73 | −8.23 | −9.01 | −8.54 | −8.43 |
| ΔS (×102 J/mol K) | 2.59 | 2.76 | 3.02 | 2.86 | 2.82 |
| Samples | Degradation Temperature (°C) | Residue at 500 °C (%) | Maximum Degradation Temperature (Tmax, °C) | |||
|---|---|---|---|---|---|---|
| Ton | T5 | T50 | T90 | |||
| NR gum | 300 | 289 | 387 | 439 | 8.53 | 379 |
| NR–CNF 1 phr | 316 | 304 | 388 | 475 | 10.08 | 381 |
| NR–CNF 2 phr | 304 | 293 | 386 | 491 | 10.50 | 378 |
| NR–CNF 3 phr | 302 | 292 | 384 | 504 | 11.20 | 378 |
| NR–CNF 4 phr | 293 | 283 | 372 | 526 | 12.67 | 364 |
| Properties | NR- gum | NR–CNF 2 phr | NR–CNF 3 phr |
|---|---|---|---|
| Tg(according to tanδ max) (°C) | −43.74 | −43.77 | −46.11 |
| Tg (according to E” max) (°C) | −53.29 | −53.11 | −55.20 |
| tanδ at 25 °C | 0.0369 | 0.0361 | 0.0368 |
| Storage Modulus (MPa) | 1.56 | 2.22 | 1.87 |
| Coefficient C | 1 | 0.83 | 0.88 |
| Volume fraction of immobilized polymer chain, (Cv) | 0 | 0.0183 | 0.0009 |
| Adhesion factor, A | 0 | −0.0011 | 0.0375 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominic C.D., M.; Joseph, R.; Begum, P.M.S.; Joseph, M.; Padmanabhan, D.; Morris, L.A.; Kumar, A.S.; Formela, K. Cellulose Nanofibers Isolated from the Cuscuta Reflexa Plant as a Green Reinforcement of Natural Rubber. Polymers 2020, 12, 814. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040814
Dominic C.D. M, Joseph R, Begum PMS, Joseph M, Padmanabhan D, Morris LA, Kumar AS, Formela K. Cellulose Nanofibers Isolated from the Cuscuta Reflexa Plant as a Green Reinforcement of Natural Rubber. Polymers. 2020; 12(4):814. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040814
Chicago/Turabian StyleDominic C.D., Midhun, Rani Joseph, P.M. Sabura Begum, Meera Joseph, Dileep Padmanabhan, Leonna Angela Morris, Athira S Kumar, and Krzysztof Formela. 2020. "Cellulose Nanofibers Isolated from the Cuscuta Reflexa Plant as a Green Reinforcement of Natural Rubber" Polymers 12, no. 4: 814. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040814