Micro Magnetic Field Produced by Fe3O4 Nanoparticles in Bone Scaffold for Enhancing Cellular Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
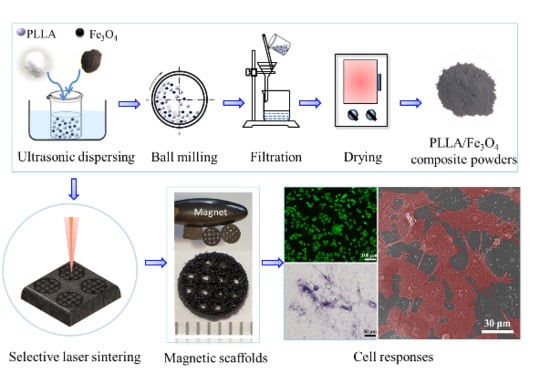
2.2. Scaffold Preparation
2.3. Characterization
2.4. Cellular Compatibility
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties and Thermal Properties
3.2. Magnetic Properties
3.3. Mechanical Properties
3.4. Cell Responses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Islami, M.; Mortazavi, Y.; Soleimani, M.; Nadri, S. In vitro expansion of CD 133+ cells derived from umbilical cord blood in poly-L-lactic acid (PLLA) scaffold coated with fibronectin and collagen. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Yusof, M.R.; Shamsudin, R.; Zakaria, S.; Abdul Hamid, M.A.; Yalcinkaya, F.; Abdullah, Y.; Yacob, N. Fabrication and characterization of carboxymethyl starch/poly (l-lactide) acid/β-tricalcium phosphate composite nanofibers via electrospinning. Polymers 2019, 11, 1468. [Google Scholar] [CrossRef] [Green Version]
- Shamsah, A.H.; Cartmell, S.H.; Richardson, S.M.; Bosworth, L.A. Material Characterization of PCL: PLLA Electrospun Fibers Following Six Months Degradation In Vitro. Polymers 2020, 12, 700. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Liu, G.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 2020, 74, 104825. [Google Scholar] [CrossRef]
- Shuai, C.; Yu, L.; Feng, P.; Zhong, Y.; Zhao, Z.; Chen, Z.; Yang, W. Organic montmorillonite produced an interlayer locking effect in a polymer scaffold to enhance interfacial bonding. Mater. Chem. Front. 2020, 4, 2398–2408. [Google Scholar] [CrossRef]
- Birhanu, G.; Akbari Javar, H.; Seyedjafari, E.; Zandi-Karimi, A.; Dusti Telgerd, M. An improved surface for enhanced stem cell proliferation and osteogenic differentiation using electrospun composite PLLA/P123 scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Castillejos, S.; Cerna, J.; Meléndez, F.; Castro, M.E.; Aguilar, R.; Márquez-Beltrán, C.; González, M. Bulk Modification of Poly (lactide)(PLA) via Copolymerization with Poly (propylene glycol) Diglycidylether (PPGDGE). Polymers 2018, 10, 1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofer, M.D.; Roessler, P.P.; Schaefer, J.; Theisen, C.; Schlimme, S.; Heverhagen, J.T.; Voelker, M.; Dersch, R.; Agarwal, S.; Fuchs-Winkelmann, S. Electrospun PLLA nanofiber scaffolds and their use in combination with BMP-2 for reconstruction of bone defects. PLoS ONE 2011, 6, e25462. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Chen, K.; Lu, W.; Sun, Q.; Peng, L.; Fen, W.; Li, H.; Ou, Y.; Liu, H.; Wang, D. In vitro study of nano-HA/PLLA composite scaffold for rabbit BMSC differentiation under TGF-β1 induction. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 214–220. [Google Scholar] [CrossRef]
- Çakır-Özkan, N.; Eğri, S.; Bekar, E.; Altunkaynak, B.Z.; Kabak, Y.B.; Kıvrak, E.G. The use of sequential VEGF-and BMP2-releasing biodegradable scaffolds in rabbit mandibular defects. J. Oral Maxillofac. Surg. 2017, 75, 221.e1–221.e14. [Google Scholar]
- Murahashi, Y.; Yano, F.; Nakamoto, H.; Maenohara, Y.; Iba, K.; Yamashita, T.; Tanaka, S.; Ishihara, K.; Okamura, Y.; Moro, T. Multi-layered PLLA-nanosheets loaded with FGF-2 induce robust bone regeneration with controlled release in critical-sized mouse femoral defects. Acta Biomater. 2019, 85, 172–179. [Google Scholar] [CrossRef]
- Hesari, R.; Keshvarinia, M.; Kabiri, M.; Rad, I.; Parivar, K.; Hoseinpoor, H.; Tavakoli, R.; Soleimani, M.; Kouhkan, F.; Zamanluee, S. Comparative impact of platelet rich plasma and transforming growth factor-β on chondrogenic differentiation of human adipose derived stem cells. Bioimpacts 2020, 10, 37. [Google Scholar] [CrossRef]
- Huang, K.-C.; Yano, F.; Murahashi, Y.; Takano, S.; Kitaura, Y.; Chang, S.H.; Soma, K.; Ueng, S.W.; Tanaka, S.; Ishihara, K. Sandwich-type PLLA-nanosheets loaded with BMP-2 induce bone regeneration in critical-sized mouse calvarial defects. Acta Biomater. 2017, 59, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Alibolandi, M.; Abnous, K.; Salmasi, Z.; Jaafari, M.R.; Ramezani, M. Fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, X.; Ding, C.; Shang, P. Effects of static magnetic fields on bone microstructure and mechanical properties in mice. Electromagn. Biol. Med. 2018, 37, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Q.; Yang, M.; Zhan, X.; Lan, F.; He, J.; Gu, Z.; Wu, Y. Protein corona of magnetic hydroxyapatite scaffold improves cell proliferation via activation of mitogen-activated protein kinase signaling pathway. ACS Nano 2017, 11, 3690–3704. [Google Scholar] [CrossRef]
- Yuan, Z.; Memarzadeh, K.; Stephen, A.S.; Allaker, R.P.; Brown, R.A.; Huang, J. Development of a 3D collagen model for the in vitro evaluation of magnetic-assisted osteogenesis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yin, G.; Huang, Z.; Deng, M.; Zeng, J.; Gu, J. Preparation and cell response of bio-mineralized Fe3O4 nanoparticles. J. Colloid Interface Sci. 2011, 363, 393–402. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Ghazanfari, M.R.; Kashefi, M.; Shams, S.F.; Jaafari, M.R. Perspective of Fe3O4 nanoparticles role in biomedical applications. Biochem. Res. Int. 2016. [Google Scholar] [CrossRef] [Green Version]
- Nehra, P.; Chauhan, R.; Garg, N.; Verma, K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br. J. Biomed. Sci. 2018, 75, 13–18. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y. Fabrication and magnetic properties of Fe3O4 nanowire arrays in different diameters. J. Magn. Magn. Mater. 2009, 321, L15–L20. [Google Scholar] [CrossRef]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Aghaie, E.; Nadernezhad, A.; Zargarzadeh, M.; Khakzad, A.; Shakeri, M.; Khosrowshahi, Y.B.; Siadati, M. Influence of Fe 3 O 4 nanoparticles in hydroxyapatite scaffolds on proliferation of primary human fibroblast cells. J. Mater. Eng. Perform. 2016, 25, 2331–2339. [Google Scholar] [CrossRef]
- Bourrinet, P.; Bengele, H.H.; Bonnemain, B.; Dencausse, A.; Idee, J.-M.; Jacobs, P.M.; Lewis, J.M. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Investig. Radiol. 2006, 41, 313–324. [Google Scholar] [CrossRef]
- Chen, L.; Peng, J.; Zhao, J.; Long, Y.; Xie, Y.; Nie, J. Magnetic Materials in Promoting Bone Regeneration. Front. Mater. 2019, 6, 268. [Google Scholar]
- Cunha, C.; Panseri, S.; Iannazzo, D.; Piperno, A.; Pistone, A.; Fazio, M.; Russo, A.; Marcacci, M.; Galvagno, S. Hybrid composites made of multiwalled carbon nanotubes functionalized with Fe3O4 nanoparticles for tissue engineering applications. Nanotechnology 2012, 23, 465102. [Google Scholar] [CrossRef]
- Shan, D.; Shi, Y.; Duan, S.; Wei, Y.; Cai, Q.; Yang, X. Electrospun magnetic poly (L-lactide)(PLLA) nanofibers by incorporating PLLA-stabilized Fe3O4 nanoparticles. Mater. Sci. Eng. C 2013, 33, 3498–3505. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, W.; Wen, X.; He, B.; Zeng, X.; Wang, G.; Gu, Z. A novel calcium phosphate ceramic–magnetic nanoparticle composite as a potential bone substitute. Biomed. Mater. 2010, 5, 015001. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Song, Y.; Han, B.; Hu, X.; Wang, X.; Lin, Y.; Deng, X. Magnetic biodegradable Fe3O4/CS/PVA nanofibrous membranes for bone regeneration. Biomed. Mater. 2011, 6, 055008. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Shi, Y.; Shan, D.; Jia, W.; Duan, S.; Deng, X.; Yang, X. Osteogenic differentiation of MC3T3-E1 cells on poly (l-lactide)/Fe3O4 nanofibers with static magnetic field exposure. Mater. Sci. Eng. C 2015, 55, 166–173. [Google Scholar] [CrossRef]
- Qin, T.; Li, X.; Long, H.; Bin, S.; Xu, Y. Bioactive Tetracalcium Phosphate Scaffolds Fabricated by Selective Laser Sintering for Bone Regeneration Applications. Materials 2020, 13, 2268. [Google Scholar] [CrossRef]
- Moqbel, N.M.; Al-Akhali, M.; Wille, S.; Kern, M. Influence of Aging on Biaxial Flexural Strength and Hardness of Translucent 3Y-TZP. Materials 2020, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Shahabadi, N.; Falsafi, M.; Mansouri, K. Improving antiproliferative effect of the anticancer drug cytarabine on human promyelocytic leukemia cells by coating on Fe3O4@ SiO2 nanoparticles. Colloids Surf. B Biointerfaces 2016, 141, 213–222. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, L.; Mak, A.F.; Ko, F.; Qin, L. Preparation and biodegradation of electrospun PLLA/keratin nonwoven fibrous membrane. Polym. Degrad. Stab. 2009, 94, 1800–1807. [Google Scholar] [CrossRef]
- Zhang, D.; Karki, A.B.; Rutman, D.; Young, D.P.; Wang, A.; Cocke, D.; Ho, T.H.; Guo, Z. Electrospun polyacrylonitrile nanocomposite fibers reinforced with Fe3O4 nanoparticles: Fabrication and property analysis. Polymer 2009, 50, 4189–4198. [Google Scholar] [CrossRef]
- Yin, G.; Zhao, D.; Ren, Y.; Zhang, L.; Zhou, Z.; Li, Q. A convenient process to fabricate gelatin modified porous PLLA materials with high hydrophilicity and strength. Biomater. Sci. 2016, 4, 310–318. [Google Scholar] [CrossRef]
- Shuai, C.; Li, Y.; Wang, G.; Yang, W.; Peng, S.; Feng, P. Surface modification of nanodiamond: Toward the dispersion of reinforced phase in poly-l-lactic acid scaffolds. Int. J. Biol. Macromol. 2019, 126, 1116–1124. [Google Scholar] [CrossRef]
- Yang, W.; Zhong, Y.; Feng, P.; Gao, C.; Peng, S.; Zhao, Z.; Shuai, C. Disperse magnetic sources constructed with functionalized Fe3O4 nanoparticles in poly-l-lactic acid scaffolds. Polym. Test. 2019, 76, 33–42. [Google Scholar] [CrossRef]
- Irez, A.; Bayraktar, E.; Miskioglu, I. Recycled and devulcanized rubber modified epoxy-based composites reinforced with nano-magnetic iron oxide, Fe3O4. Compos. Part. B Eng. 2018, 148, 1–13. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, B.; Bin, S.; Peng, S.; Gao, C. TiO2 induced in situ reaction in graphene oxide reinforced AZ61 biocomposites to enhance the interfacial bonding. ACS Appl. Mater. Interfaces 2020, 12, 23464–23473. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yu, L.; Feng, P.; Gao, C.; Peng, S. Interfacial reinforcement in bioceramic/biopolymer composite bone scaffold: The role of coupling agent. Colloids Surf. B Biointerfaces 2020, 193, 111083. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Li, Y.; Zou, Q. Degradation and biocompatibility of porous nano-hydroxyapatite/polyurethane composite scaffold for bone tissue engineering. Appl. Surf. Sci. 2009, 255, 6087–6091. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Mohammadi-Aghdam, M.; Saber-Samandari, S. A novel magnetic bifunctional nanocomposite scaffold for photothermal therapy and tissue engineering. Int. J. Biol. Macromol. 2019, 138, 810–818. [Google Scholar] [CrossRef]
- Blank, M. Protein and DNA reactions stimulated by electromagnetic fields. Electr. Magn. Biol. Med. 2008, 27, 3. [Google Scholar] [CrossRef] [PubMed]
- Cifra, M.; Fields, J.Z.; Farhadi, A. Electromagnetic cellular interactions. Prog. Biophys. Mol. Biol. 2011, 105, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, T.; Yamaguchi, H.; Miyamoto, H. Effect of electromagnetic fields on membrane ion transport of cultured cells. J. Med. Investig. 1998, 45, 47–56. [Google Scholar]
- Yan, X.; Song, G.; Wang, X.; Zhao, Y.; Chen, Y. The preparation and medical applications of chitosan microspheres. Curr. Org. Chem. 2018, 22, 720–733. [Google Scholar] [CrossRef]
- Adey, W.R. Biological effects of electromagnetic fields. J. Cell. Biochem. 1993, 51, 410–416. [Google Scholar] [CrossRef]
- Gorgun, S.S. Studies on the interaction between electromagnetic fields and living matter neoplastic cellular culture. Cent. Front. Sci. Thmple Univ. 1998, 7, 1–21. [Google Scholar]
- Huang, D.-M.; Hsiao, J.-K.; Chen, Y.-C.; Chien, L.-Y.; Yao, M.; Chen, Y.-K.; Ko, B.-S.; Hsu, S.-C.; Tai, L.-A.; Cheng, H.-Y. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.A.; Syrovets, T.; Hafner, S.; Zablotskii, V.; Dejneka, A.; Simmet, T. Spatiotemporal magnetic fields enhance cytosolic Ca2+ levels and induce actin polymerization via activation of voltage-gated sodium channels in skeletal muscle cells. Biomaterials 2018, 163, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Kunze, A.; Murray, C.; Di Carlo, D. Induction of calcium influx in cortical neural networks by nanomagnetic forces. Acs Nano 2016, 10, 2331–2341. [Google Scholar] [CrossRef]
- Yap, J.L.Y.; Tai, Y.K.; Fröhlich, J.; Fong, C.H.H.; Yin, J.N.; Foo, Z.L.; Ramanan, S.; Beyer, C.; Toh, S.J.; Casarosa, M. Ambient and supplemental magnetic fields promote myogenesis via a TRPC1—mitochondrial axis: Evidence of a magnetic mitohormetic mechanism. FASEB J. 2019, 33, 12853–12872. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Du, J.; Song, W.; Cao, M.; Chen, S.; Xia, R. Weak power frequency magnetic fields induce microtubule cytoskeleton reorganization depending on the epidermal growth factor receptor and the calcium related signaling. PLoS ONE 2018, 13, e0205569. [Google Scholar] [CrossRef]
- Lew, W.Z.; Feng, S.W.; Lin, C.T.; Huang, H.M. Use of 0.4—Tesla static magnetic field to promote reparative dentine formation of dental pulp stem cells through activation of p38 MAPK signalling pathway. Int. Endod. J. 2019, 52, 28–43. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.-M.; Kang, S.-K.; Singh, R.K.; Lee, J.-H.; Lee, H.-H.; Park, K.-R.; Yi, J.-K.; Lee, D.-W.; Kim, H.-W.; Kim, E.-C. Magnetic nanofiber scaffold-induced stimulation of odontogenesis and pro-angiogenesis of human dental pulp cells through Wnt/MAPK/NF-κB pathways. Dent. Mater. 2016, 32, 1301–1311. [Google Scholar] [CrossRef]
- Li, W.; Zhao, S.; He, W.; Zhang, M.; Li, S.; Xu, Y. Static magnetic fields accelerate osteogenesis by regulating FLRT/BMP pathway. Biochem. Biophys. Res. Commun. 2020, 527, 83–89. [Google Scholar] [CrossRef]
- Yang, W.; Zhong, Y.; He, C.; Peng, S.; Yang, Y.; Qi, F.; Feng, P.; Shuai, C. Electrostatic self-assembly of pFe3O4 nanoparticles on graphene oxide: A co-dispersed nanosystem reinforces PLLA scaffolds. J. Adv. Res. 2020, 24, 191–203. [Google Scholar] [CrossRef]
- Long, L.; Yuan, Z.; Yin, L.P.; Liu, R.; Huang, B.L.; Liu, S.Y. An observation on effect of pure magnetic nanoparticles (Fe3O4) on human hepatoma cells and human lung adenocarcinoma cells. J. Toxicol. 2009, 23, 89–92. [Google Scholar]
- Huang, X.; Ma, P.; Rao, Y.; Zhao, W.; Yang, X. Pulmonary biosafety of Fe3O4 nanoparticles used in sports engineering on Kunming mice. Ferroelectrics 2018, 527, 44–51. [Google Scholar] [CrossRef]
- Park, E.J.; Umh, H.N.; Choi, D.H.; Cho, M.H.; Choi, W.; Kim, S.W.; Kim, Y.; Kim, J.H. Magnetite- and maghemite-induced different toxicity in murine alveolar macrophage cells. Arch. Toxicol. 2014, 88, 1607–1618. [Google Scholar] [CrossRef]
- Wojasiński, M.; Faliszewski, K.; Ciach, T. Electrospinning production of PLLA fibrous scaffolds for tissue engineering. Chall. Mod. Technol. 2013, 4, 9–15. [Google Scholar]
- Chen, H.M.; Feng, C.X.; Zhang, W.B.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y. Hydrolytic degradation behavior of poly (l-lactide)/carbon nanotubes nanocomposites. Polym. Degrad. Stab. 2013, 98, 198–208. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin, S.; Wang, A.; Guo, W.; Yu, L.; Feng, P. Micro Magnetic Field Produced by Fe3O4 Nanoparticles in Bone Scaffold for Enhancing Cellular Activity. Polymers 2020, 12, 2045. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092045
Bin S, Wang A, Guo W, Yu L, Feng P. Micro Magnetic Field Produced by Fe3O4 Nanoparticles in Bone Scaffold for Enhancing Cellular Activity. Polymers. 2020; 12(9):2045. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092045
Chicago/Turabian StyleBin, Shizhen, Ailun Wang, Wang Guo, Li Yu, and Pei Feng. 2020. "Micro Magnetic Field Produced by Fe3O4 Nanoparticles in Bone Scaffold for Enhancing Cellular Activity" Polymers 12, no. 9: 2045. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092045